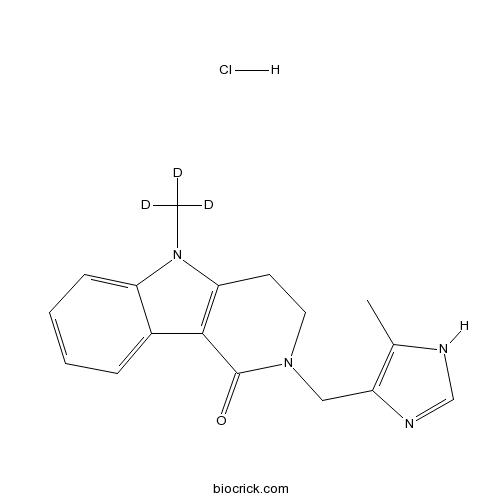
Alosetron-d3 HydrochlorideCAS# 1189919-71-8 |
- Dihydroberberine
Catalog No.:BCN2573
CAS No.:483-15-8
- Pectolinarigenin
Catalog No.:BCN5813
CAS No.:520-12-7
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- Hypaconine
Catalog No.:BCN8640
CAS No.:63238-68-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1189919-71-8 | SDF | Download SDF |
| PubChem ID | 46780129 | Appearance | Powder |
| Formula | C17H16D3ClN4O | M.Wt | 333.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-[(5-methyl-1H-imidazol-4-yl)methyl]-5-(trideuteriomethyl)-3,4-dihydropyrido[4,3-b]indol-1-one;hydrochloride | ||
| SMILES | CC1=C(N=CN1)CN2CCC3=C(C2=O)C4=CC=CC=C4N3C.Cl | ||
| Standard InChIKey | FNYQZOVOVDSGJH-MUTAZJQDSA-N | ||
| Standard InChI | InChI=1S/C17H18N4O.ClH/c1-11-13(19-10-18-11)9-21-8-7-15-16(17(21)22)12-5-3-4-6-14(12)20(15)2;/h3-6,10H,7-9H2,1-2H3,(H,18,19);1H/i2D3; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Alosetron-d3 Hydrochloride Dilution Calculator

Alosetron-d3 Hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9955 mL | 14.9777 mL | 29.9554 mL | 59.9107 mL | 74.8884 mL |
| 5 mM | 0.5991 mL | 2.9955 mL | 5.9911 mL | 11.9821 mL | 14.9777 mL |
| 10 mM | 0.2996 mL | 1.4978 mL | 2.9955 mL | 5.9911 mL | 7.4888 mL |
| 50 mM | 0.0599 mL | 0.2996 mL | 0.5991 mL | 1.1982 mL | 1.4978 mL |
| 100 mM | 0.03 mL | 0.1498 mL | 0.2996 mL | 0.5991 mL | 0.7489 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Alosetron is s Serotonin 5HT3-receptor antagonist that is used in treatment of irritable bowel syndrome.
- Neohesperidin
Catalog No.:BCN5915
CAS No.:13241-33-3
- Neoeriocitrin
Catalog No.:BCN3271
CAS No.:13241-32-2
- Chrysophanol 8-O-glucoside
Catalog No.:BCN6175
CAS No.:13241-28-6
- β-D-Glucopyranoside,(3β,6α,16β,20R,24S)-3-[(3,4-di-O-acetyl-β-D-xylopyranosyl)oxy]-20, 24-epoxy-16,25-dihydroxy-9,19-cyclolanostan-6-yl
Catalog No.:BCC8370
CAS No.:1324005-51-7
- TC-G 1006
Catalog No.:BCC6277
CAS No.:1324003-64-6
- Boc-Gln(Trt)-OH
Catalog No.:BCC3384
CAS No.:132388-69-3
- Boc-Asn(Trt)-OH
Catalog No.:BCC3360
CAS No.:132388-68-2
- Fmoc-Gln(Trt)-OPfp
Catalog No.:BCC3486
CAS No.:132388-65-9
- Fmoc-Asn(Trt)-OPfp
Catalog No.:BCC3082
CAS No.:132388-64-8
- Z-Gln(Trt)-OH
Catalog No.:BCC2782
CAS No.:132388-60-4
- Fmoc-Asn(Trt)-OH
Catalog No.:BCC3081
CAS No.:132388-59-1
- H-Asn(Trt)-OH
Catalog No.:BCC2878
CAS No.:132388-58-0
- 2-TEDC
Catalog No.:BCC6735
CAS No.:132465-10-2
- (Z)-Pugnac
Catalog No.:BCC5333
CAS No.:132489-69-1
- Olanzapine
Catalog No.:BCC5042
CAS No.:132539-06-1
- 15-Demethylplumieride
Catalog No.:BCN6176
CAS No.:132586-69-7
- Bruceanic acid C
Catalog No.:BCN7999
CAS No.:132587-60-1
- CCMQ
Catalog No.:BCC6984
CAS No.:132623-44-0
- Otophylloside O
Catalog No.:BCN7337
CAS No.:1326583-08-7
- Senecionine N-oxide
Catalog No.:BCN2130
CAS No.:13268-67-2
- Fmoc-Tle-OH
Catalog No.:BCC2657
CAS No.:132684-60-7
- [Leu31,Pro34]-Neuropeptide Y (human, rat)
Catalog No.:BCC5722
CAS No.:132699-73-1
- PyBrOP
Catalog No.:BCC2821
CAS No.:132705-51-2
- CP 96345
Catalog No.:BCC7509
CAS No.:132746-60-2
Alosetron, a 5-HT3 receptor antagonist, delays colonic transit in patients with irritable bowel syndrome and healthy volunteers.[Pubmed:10848662]
Aliment Pharmacol Ther. 2000 Jun;14(6):775-82.
BACKGROUND: Alosetron is a potent and selective 5-HT3 receptor antagonist, which has been shown to be beneficial in the treatment of female patients with non-constipated irritable bowel syndrome. AIMS: To investigate the effect of alosetron on whole gut, small bowel and colonic transit in patients with irritable bowel syndrome (Study 1) and healthy volunteers (Study 2). SUBJECTS: Thirteen patients with irritable bowel syndrome and 12 healthy volunteers. METHODS: Both studies were randomized, double-blind, placebo-controlled with a two-way crossover design, in which each subject received alosetron (2 mg b.d. administered orally) or placebo for 8 days. Mean whole gut transit was determined from the excretion of radio-opaque markers; small bowel transit was determined from rise in breath hydrogen after a meal; and colonic transit and segmental transit were evaluated from abdominal X-ray. In addition, colonic transit was calculated by subtracting small bowel transit time from whole gut transit time. RESULTS: Alosetron increased colonic transit time by prolonging left colonic transit in both patients with irritable bowel syndrome and controls. This resulted in a tendency for the whole gut transit to be delayed in irritable bowel syndrome patients (P=0.128), which was confirmed in controls (P=0.047). CONCLUSION: Alosetron delays colonic transit by prolonging left colonic transit. These results add to the body of evidence suggesting that alosetron should have a therapeutic role in patients with non-constipated irritable bowel syndrome.
Application of a UPLC-MS/MS method for the analysis of alosetron in human plasma to support a bioequivalence study in healthy males and females.[Pubmed:25761551]
Biomed Chromatogr. 2015 Oct;29(10):1527-34.
A simple, rapid and sensitive ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method has been developed and validated for the determination of alosetron (ALO) in human plasma. The assay method involved solid-phase extraction of ALO and ALO 13C-d3 as internal standard (IS) on a LichroSep DVB-HL (30 mg, 1 cm(3) ) cartridge. The chromatography was performed on an Acquity UPLC BEH C18 (50 x 2.1 mm, 1.7 microm) column using acetonitrile and 2.0 mm ammonium formate, pH 3.0 adjusted with 0.1% formic acid (80:20, v/v) as the mobile phase in an isocratic mode. For quantitative analysis, the multiple reaction monitoring transitions studied were m/z 295.1/201.0 for ALO and m/z 299.1/205.1 for IS in the positive ionization mode. The method was validated over a concentration range of 0.01-10.0 ng/mL for ALO. Post-column infusion experiment showed no positive or negative peaks in the elution range of the analyte and IS after injection of extracted blank plasma. The extent of ion-suppression/enhancement, expressed as IS-normalized matrix factor, varied from 0.96 to 1.04. The assay recovery was within 97-103% for ALO and IS. The method was successfully applied to support a bioequivalence study of 1.0 mg alosetron tablets in 28 healthy Indian male and female subjects.
Development of a Forced Degradation Profile of Alosetron by Single Mode Reversed-Phase HPLC, LC-MS, and its Validation.[Pubmed:26839817]
Sci Pharm. 2014 Dec 29;83(2):311-20.
Determination of alosetron in the presence of its degradation products was studied and validated by a novel HPLC method. The separation of the drug and its degradation products was achieved with the Jones Chromatography C18 analytical column (150 mm x 4.6 mm; 3 microm) with a stationary phase in isocratic elution mode. The mobile phase used was 0.01 M ammonium acetate, pH-adjusted to 3.5 with glacial acetic acid and acetonitrile in the ratio of 75:25 (V/V) at a flow rate of 1 ml/min and UV detection was carried out at 217 nm. Further, the drug was subjected to stress studies for acidic, basic, neutral, oxidative, and thermal degradations as per ICH guidelines and the drug was found to be labile in base hydrolysis and oxidation, while stable in acid, neutral, thermal, and photolytic degradation conditions. An MS study has been performed on the major degradation products to predict the degradation pathway of alosetron. The method provided linear responses over the concentration range of 100-1500 ng/ml and regression analysis showed a correlation coefficient value (r(2)) of 0.994. The LOD and LOQ were found to be 1 ng/ml and 3 ng/ml, respectively. The developed LC method was validated as per ICH guidelines with respect to accuracy, selectivity, precision, linearity, and robustness.


