Fmoc-Asn(Trt)-OHCAS# 132388-59-1 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 132388-59-1 | SDF | Download SDF |
| PubChem ID | 7168041 | Appearance | Powder |
| Formula | C38H32N2O5 | M.Wt | 596.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water or 1% acetic acid | ||
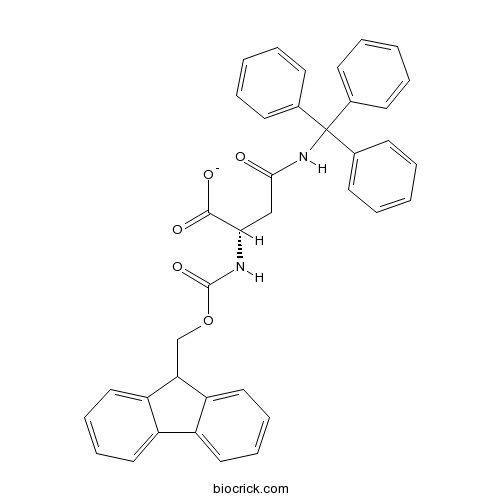
| Chemical Name | (2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-4-oxo-4-(tritylamino)butanoate | ||
| SMILES | C1=CC=C(C=C1)C(C2=CC=CC=C2)(C3=CC=CC=C3)NC(=O)CC(C(=O)[O-])NC(=O)OCC4C5=CC=CC=C5C6=CC=CC=C46 | ||
| Standard InChIKey | KJYAFJQCGPUXJY-UMSFTDKQSA-M | ||
| Standard InChI | InChI=1S/C38H32N2O5/c41-35(40-38(26-14-4-1-5-15-26,27-16-6-2-7-17-27)28-18-8-3-9-19-28)24-34(36(42)43)39-37(44)45-25-33-31-22-12-10-20-29(31)30-21-11-13-23-32(30)33/h1-23,33-34H,24-25H2,(H,39,44)(H,40,41)(H,42,43)/p-1/t34-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fmoc-Asn(Trt)-OH Dilution Calculator

Fmoc-Asn(Trt)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6759 mL | 8.3794 mL | 16.7588 mL | 33.5177 mL | 41.8971 mL |
| 5 mM | 0.3352 mL | 1.6759 mL | 3.3518 mL | 6.7035 mL | 8.3794 mL |
| 10 mM | 0.1676 mL | 0.8379 mL | 1.6759 mL | 3.3518 mL | 4.1897 mL |
| 50 mM | 0.0335 mL | 0.1676 mL | 0.3352 mL | 0.6704 mL | 0.8379 mL |
| 100 mM | 0.0168 mL | 0.0838 mL | 0.1676 mL | 0.3352 mL | 0.419 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-Asn(Trt)-OH
- H-Asn(Trt)-OH
Catalog No.:BCC2878
CAS No.:132388-58-0
- 10-Hydroxyneoline
Catalog No.:BCN6510
CAS No.:132362-42-6
- 5-Acetoxy-7-hydroxyflavone
Catalog No.:BCN6174
CAS No.:132351-58-7
- Methyl isocostate
Catalog No.:BCN6173
CAS No.:132342-55-3
- 4-Epialyxialactone
Catalog No.:BCN6172
CAS No.:132339-37-8
- Benzyl ferulate
Catalog No.:BCN7753
CAS No.:132335-97-8
- Fmoc-Gln(Trt)-OH
Catalog No.:BCC3485
CAS No.:132327-80-1
- Hemiphroside B nonaacetate
Catalog No.:BCN7336
CAS No.:132302-25-1
- Salpriolactone
Catalog No.:BCN3220
CAS No.:132278-72-9
- Rubioncolin C
Catalog No.:BCN7871
CAS No.:132242-52-5
- Alyxialactone
Catalog No.:BCN6171
CAS No.:132237-63-9
- 3,6-Dibenzyl-2-hydroxy-5-methoxypyrazine
Catalog No.:BCN7335
CAS No.:132213-65-1
- Z-Gln(Trt)-OH
Catalog No.:BCC2782
CAS No.:132388-60-4
- Fmoc-Asn(Trt)-OPfp
Catalog No.:BCC3082
CAS No.:132388-64-8
- Fmoc-Gln(Trt)-OPfp
Catalog No.:BCC3486
CAS No.:132388-65-9
- Boc-Asn(Trt)-OH
Catalog No.:BCC3360
CAS No.:132388-68-2
- Boc-Gln(Trt)-OH
Catalog No.:BCC3384
CAS No.:132388-69-3
- TC-G 1006
Catalog No.:BCC6277
CAS No.:1324003-64-6
- β-D-Glucopyranoside,(3β,6α,16β,20R,24S)-3-[(3,4-di-O-acetyl-β-D-xylopyranosyl)oxy]-20, 24-epoxy-16,25-dihydroxy-9,19-cyclolanostan-6-yl
Catalog No.:BCC8370
CAS No.:1324005-51-7
- Chrysophanol 8-O-glucoside
Catalog No.:BCN6175
CAS No.:13241-28-6
- Neoeriocitrin
Catalog No.:BCN3271
CAS No.:13241-32-2
- Neohesperidin
Catalog No.:BCN5915
CAS No.:13241-33-3
- Alosetron-d3 Hydrochloride
Catalog No.:BCC1345
CAS No.:1189919-71-8
- 2-TEDC
Catalog No.:BCC6735
CAS No.:132465-10-2
A 'conovenomic' analysis of the milked venom from the mollusk-hunting cone snail Conus textile--the pharmacological importance of post-translational modifications.[Pubmed:24055806]
Peptides. 2013 Nov;49:145-58.
Cone snail venoms provide a largely untapped source of novel peptide drug leads. To enhance the discovery phase, a detailed comparative proteomic analysis was undertaken on milked venom from the mollusk-hunting cone snail, Conus textile, from three different geographic locations (Hawai'i, American Samoa and Australia's Great Barrier Reef). A novel milked venom conopeptide rich in post-translational modifications was discovered, characterized and named alpha-conotoxin TxIC. We assign this conopeptide to the 4/7 alpha-conotoxin family based on the peptide's sequence homology and cDNA pre-propeptide alignment. Pharmacologically, alpha-conotoxin TxIC demonstrates minimal activity on human acetylcholine receptor models (100 muM, <5% inhibition), compared to its high paralytic potency in invertebrates, PD50 = 34.2 nMol kg(-1). The non-post-translationally modified form, [Pro](2,8)[Glu](16)alpha-conotoxin TxIC, demonstrates differential selectivity for the alpha3beta2 isoform of the nicotinic acetylcholine receptor with maximal inhibition of 96% and an observed IC50 of 5.4 +/- 0.5 muM. Interestingly its comparative PD50 (3.6 muMol kg(-1)) in invertebrates was ~100 fold more than that of the native peptide. Differentiating alpha-conotoxin TxIC from other alpha-conotoxins is the high degree of post-translational modification (44% of residues). This includes the incorporation of gamma-carboxyglutamic acid, two moieties of 4-trans hydroxyproline, two disulfide bond linkages, and C-terminal amidation. These findings expand upon the known chemical diversity of alpha-conotoxins and illustrate a potential driver of toxin phyla-selectivity within Conus.
Novel N omega-xanthenyl-protecting groups for asparagine and glutamine, and applications to N alpha-9-fluorenylmethyloxycarbonyl (Fmoc) solid-phase peptide synthesis.[Pubmed:8914163]
Pept Res. 1996 Jul-Aug;9(4):166-73.
The N alpha-9-fluorenylmethyloxycarbonyl (Fmoc), N omega-9H-xanthen-9-yl (Xan), N omega-2-methoxy-9H-xanthen-9-yl (2-Moxan) or N omega-3-methoxy-9H-xanthen-9-yl (3-Moxan) derivatives of asparagine and glutamine were prepared conveniently by acid-catalyzed reactions of appropriate xanthydrols with Fmoc-Asn-OH and Fmoc-Gln-OH. The Xan and 2-Moxan protected derivatives have been used in Fmoc solid-phase syntheses of several challenging peptides: a modified Riniker's peptide to probe tryptophanalkylation side reactions, Briand's peptide to assess deblocking, at the N-terminus and Marshall's ACP (65-74) to test difficult couplings. Removal of the Asn and Gln side-chain protection occurred concomitantly with release of peptide from the support, under the conditions for acidolytic cleavage of the tris(alkoxy)benzylamide (PAL) anchoring linkage by use of trifluoroacetic acid/scavenger mixtures. For each of the model peptides, the products obtained by the new protection schemes were purer than those obtained with N omega-2,4,6-trimethoxybenzyl (Tmob) or N omega-triphenylmethyl (Trt) protection for Asn and Gln.
Multiple synthesis by the multipin method as a methodological tool.[Pubmed:9222986]
J Pept Sci. 1995 Jan-Feb;1(1):80-7.
The multipin method of peptide synthesis is demonstrated as a potent methodological tool, where large numbers of comparative studies can be performed concurrently. Two studies are presented. In each study, the test peptides were simultaneously synthesized, and the products examined by high throughput ion spray mass spectrometry and reverse-phase HPLC. In the first study, comprising 24 experiments, peptides 1 (AELFSTHYLAFKEDYSQ-NH2) and 2 (LKDFRVYFREGRDQLWKGPG-NH2) were prepared using Fmoc-Axx/BOP/HOBt/NMM [100 : 100 : 100 : 150 mM) and Fmoc-AXX/HATU/HOAt/NMM (100 : 100 : 100 : 150 nM) with 60, 90 and 120 min coupling times. The two reagent combinations were found to give comparable results. The second study compared the N-terminal coupling of Fmoc-Asn-OH, Fmoc-Asn(Mbh)-OH, Fmoc-Asn(Mtt)-OH, Fmoc-Asn(Tmob)-OH and Fmoc-Asn(Trt)-OH in the synthesis of seven test peptides: 3, NVQAAIDYIG-cyclo(KP): 4. NTVQAAIDYIG-cyclo(KP): 5. NRVYVHPFNL: 6. NRVYVHPFHL: 7. NEAYVHDAPVRSLN: 8. NQLVVPSEGLYLIYSQVLFK; 9, NPNANPNANPNA. A total of 33 experiments were performed. Peptides 3 and 4 were selected to highlight the effect of steric bulk of each Asn derivative on coupling efficiency. Reagent efficiency, as measured by target peptide purity, was as follows: Fmoc-Asn(Tmob)-OH > Fmoc-Asn-OH > Fmoc-Asn(Mtt)-OH = Fmoc-Asn(Trt)-OH > Fmoc-Asn(Mbh)-OH.


