1,4,5,6-Tetrahydroxy-7-prenylxanthoneCAS# 1001424-68-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1001424-68-5 | SDF | Download SDF |
| PubChem ID | 71307363 | Appearance | Yellow powder |
| Formula | C18H16O6 | M.Wt | 328.3 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
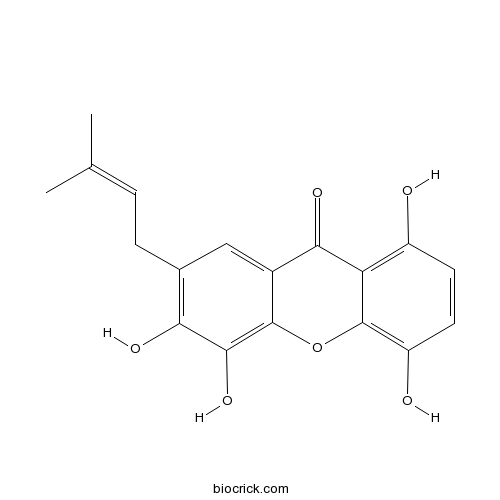
| Chemical Name | 1,4,5,6-tetrahydroxy-7-(3-methylbut-2-enyl)xanthen-9-one | ||
| SMILES | CC(=CCC1=C(C(=C2C(=C1)C(=O)C3=C(C=CC(=C3O2)O)O)O)O)C | ||
| Standard InChIKey | FPOOJYQXDQYQKU-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 1,4,5,6-Tetrahydroxy-7-prenylxanthone exhibits moderate activities with GI50 (Growth inhibitory) values of 2.8 μM against the human leukaemic HL-60 cell line were measured in vitro. 2. 1,4,5,6-Tetrahydroxy-7-prenylxanthone shows moderate cytotoxicities against breast cancer (MDA-MB-435S) and lung adenocarcinoma (A549) cell lines. |

1,4,5,6-Tetrahydroxy-7-prenylxanthone Dilution Calculator

1,4,5,6-Tetrahydroxy-7-prenylxanthone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.046 mL | 15.23 mL | 30.4599 mL | 60.9199 mL | 76.1499 mL |
| 5 mM | 0.6092 mL | 3.046 mL | 6.092 mL | 12.184 mL | 15.23 mL |
| 10 mM | 0.3046 mL | 1.523 mL | 3.046 mL | 6.092 mL | 7.615 mL |
| 50 mM | 0.0609 mL | 0.3046 mL | 0.6092 mL | 1.2184 mL | 1.523 mL |
| 100 mM | 0.0305 mL | 0.1523 mL | 0.3046 mL | 0.6092 mL | 0.7615 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PDK1 inhibitor
Catalog No.:BCC1843
CAS No.:1001409-50-2
- VUF 8430 dihydrobromide
Catalog No.:BCC7384
CAS No.:100130-32-3
- GDC-0068 (RG7440)
Catalog No.:BCC1271
CAS No.:1001264-89-6
- GnRH Associated Peptide (GAP) (1-13), human
Catalog No.:BCC1013
CAS No.:100111-07-7
- Chloramultilide D
Catalog No.:BCN7102
CAS No.:1000995-49-2
- Chloramultilide C
Catalog No.:BCN6618
CAS No.:1000995-48-1
- Chloramultilide B
Catalog No.:BCN6613
CAS No.:1000995-47-0
- H-Lys(Tfa)-OH
Catalog No.:BCC2985
CAS No.:10009-20-8
- 2',4'-Dihydroxy-2,3',6'-trimethoxychalcone
Catalog No.:BCN1643
CAS No.:100079-39-8
- 7-Hydroxy-2',5,8-trimethoxyflavanone
Catalog No.:BCN5817
CAS No.:100079-34-3
- Tegobuvir
Catalog No.:BCC1991
CAS No.:1000787-75-6
- Stilbostemin N
Catalog No.:BCN4741
CAS No.:1000676-45-8
- BV6
Catalog No.:BCC5372
CAS No.:1001600-56-1
- SRT1720 HCl
Catalog No.:BCC2222
CAS No.:1001645-58-4
- INH6
Catalog No.:BCC5455
CAS No.:1001753-24-7
- MK-8033
Catalog No.:BCC1768
CAS No.:1001917-37-8
- Piscidinol A
Catalog No.:BCN5818
CAS No.:100198-09-2
- 2,3-dihydroxy-3-(4-hydroxyphenyl)propanoic acid
Catalog No.:BCN1641
CAS No.:100201-57-8
- 5,5'-Dimethoxysecoisolariciresinol
Catalog No.:BCN7941
CAS No.:1002106-91-3
- Camstatin
Catalog No.:BCC5690
CAS No.:1002295-95-5
- AMG-208
Catalog No.:BCC1054
CAS No.:1002304-34-8
- Picrasidine I
Catalog No.:BCN5819
CAS No.:100234-59-1
- Picrasidine J
Catalog No.:BCN5820
CAS No.:100234-62-6
- TZ9
Catalog No.:BCC5547
CAS No.:1002789-86-7
Xanthone biosynthesis in Hypericum perforatum cells provides antioxidant and antimicrobial protection upon biotic stress.[Pubmed:19062051]
Phytochemistry. 2009 Jan;70(1):60-8.
Xanthone production in Hypericum perforatum (HP) suspension cultures in response to elicitation by Agrobacterium tumefaciens co-cultivation has been studied. RNA blot analyses of HP cells co-cultivated with A. tumefaciens have shown a rapid up-regulation of genes encoding important enzymes of the general phenylpropanoid pathway (PAL, phenylalanine ammonia lyase and 4CL, 4-coumarate:CoA ligase) and xanthone biosynthesis (BPS, benzophenone synthase). Analyses of HPLC chromatograms of methanolic extracts of control and elicited cells (HP cells that were co-cultivated for 24h with A. tumefaciens) have revealed a 12-fold increase in total xanthone concentration and also the emergence of many xanthones after elicitation. Methanolic extract of elicited cells exhibited significantly higher antioxidant and antimicrobial competence than the equivalent extract of control HP cells indicating that these properties have been significantly increased in HP cells after elicitation. Four major de novo synthesized xanthones have been identified as 1,3,6,7-tetrahydroxy-8-prenyl xanthone, 1,3,6,7-tetrahydroxy-2-prenyl xanthone, 1,3,7-trihydroxy-6-methoxy-8-prenyl xanthone and paxanthone. Antioxidant and antimicrobial characterization of these de novo xanthones have revealed that xanthones play dual function in plant cells during biotic stress: (1) as antioxidants to protect the cells from oxidative damage and (2) as phytoalexins to impair the pathogen growth.


