MK-8033C-MET inhibitor CAS# 1001917-37-8 |
- AMG-208
Catalog No.:BCC1054
CAS No.:1002304-34-8
- SGX-523
Catalog No.:BCC1055
CAS No.:1022150-57-7
- PHA-665752
Catalog No.:BCC1181
CAS No.:477575-56-7
- SU11274
Catalog No.:BCC1243
CAS No.:658084-23-2
- Cabozantinib (XL184, BMS-907351)
Catalog No.:BCC1264
CAS No.:849217-68-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1001917-37-8 | SDF | Download SDF |
| PubChem ID | 45142457 | Appearance | Powder |
| Formula | C25H21N5O3S | M.Wt | 471.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 46 mg/mL (97.55 mM) *"≥" means soluble, but saturation unknown. | ||
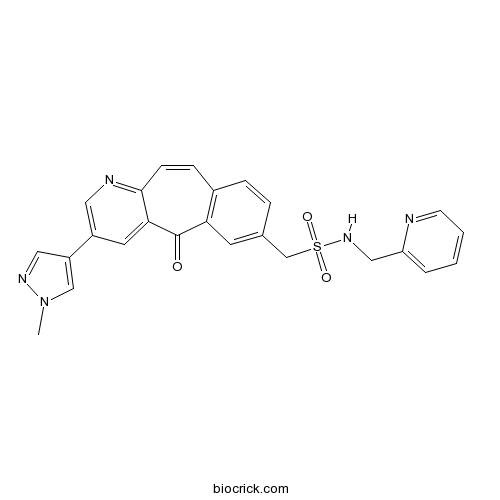
| Chemical Name | 1-[2-(1-methylpyrazol-4-yl)-11-oxobenzo[1,2]cyclohepta[2,4-b]pyridin-9-yl]-N-(pyridin-2-ylmethyl)methanesulfonamide | ||
| SMILES | CN1C=C(C=N1)C2=CN=C3C=CC4=C(C=C(C=C4)CS(=O)(=O)NCC5=CC=CC=N5)C(=O)C3=C2 | ||
| Standard InChIKey | VMJFTOSOFDEKTM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H21N5O3S/c1-30-15-20(13-28-30)19-11-23-24(27-12-19)8-7-18-6-5-17(10-22(18)25(23)31)16-34(32,33)29-14-21-4-2-3-9-26-21/h2-13,15,29H,14,16H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | MK8033 is a novel and specific dual ATP competitive c-Met/Ron inhibitor (IC50=1 nM Wt c-Met) under investigation as a treatment for cancer.
IC50 Value: 1 nM (Wt c-Met); 2.0 nM (c-Met N1100Y) [1]
Target: c-Met/Ron
in vitro: MK-8033 binds 3-fold more tightly to phosphorylated c-Met kinase domain (Kd= 3.2 nM) than to its unphosphorylated counterpart (Kd = 10.4 nM). Signigicantly, MK-8033 potently inhibits kinase activity of three oncogenic c-Met activation loop mutants, Y1230C, Y1230H, and Y1235D (IC50s ranging from 0.6 to 1 nM at 50 uM ATP) in addition to other c-Met activating mutants N1100Y and M1250T. MK-8033 potently inhibited GTL-16 proliferation with an IC50 of 582 ± 30 nM. By contrast the HCT116 cell line, which does not harbor basal c-Met activation, was not inhibited by MK-8033 (IC50 > 10000 nM) [1]. MK-8033 radiosensitized the high-c-Met-expressing EBC-1 and H1993 cells but not the low-c-Met-expressing cell lines A549 and H460. However, irradiation of A549 and H460 cells increased the expression of c-Met protein at 30 minutes after the irradiation. Subsequent targeting of this up-regulated c-Met by using MK-8033 followed by a second radiation dose reduced the clonogenic survival of both A549 and H460 cells. MK-8033reduced the levels of radiation-induced phosphorylated (activated) c-Met in A549 cells [2].
in vivo: MK-8033 was orally dosed in GTL-16 tumor xenograft bearing mice. Mice were euthanized 1 h after dosing and tested for p-Met (Y1349) in tumors and MK-8033 concentrations in plasma. At 100 mg/kg,essentially complete inhibition of p-Met (Y1349) was achieved. An in vivo IC50 of 1.3 uM was deduced from the relationship between plasma MK-8033 level and Met pY1349. Treatment with escalating dosed of MK-8033 for 21 days lead to antitumor efficacies in a dose-dependent manner. Dosing at 3, 10, 30, and 100 mg/kg resulted in 22, 18, 57, and 86% tumor growth inhibition, respectively, relative to tumor from vehicle-treated mice.
signatures. References: | |||||

MK-8033 Dilution Calculator

MK-8033 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1208 mL | 10.6038 mL | 21.2076 mL | 42.4151 mL | 53.0189 mL |
| 5 mM | 0.4242 mL | 2.1208 mL | 4.2415 mL | 8.483 mL | 10.6038 mL |
| 10 mM | 0.2121 mL | 1.0604 mL | 2.1208 mL | 4.2415 mL | 5.3019 mL |
| 50 mM | 0.0424 mL | 0.2121 mL | 0.4242 mL | 0.8483 mL | 1.0604 mL |
| 100 mM | 0.0212 mL | 0.106 mL | 0.2121 mL | 0.4242 mL | 0.5302 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MK-8033 is a c-MET inhibitor in clinical trials. MK8033 is a selective small-molecule inhibitor, ATP competitive. Phase I investigation of the cMet inhibitor MK-8033 is ongoing. Plans include a trial of this agent in refractory colorectal cancer, with pre- and post-treatment biopsies to evaluate for relevant molecular signatures.
- INH6
Catalog No.:BCC5455
CAS No.:1001753-24-7
- SRT1720 HCl
Catalog No.:BCC2222
CAS No.:1001645-58-4
- BV6
Catalog No.:BCC5372
CAS No.:1001600-56-1
- 1,4,5,6-Tetrahydroxy-7-prenylxanthone
Catalog No.:BCN1642
CAS No.:1001424-68-5
- PDK1 inhibitor
Catalog No.:BCC1843
CAS No.:1001409-50-2
- VUF 8430 dihydrobromide
Catalog No.:BCC7384
CAS No.:100130-32-3
- GDC-0068 (RG7440)
Catalog No.:BCC1271
CAS No.:1001264-89-6
- GnRH Associated Peptide (GAP) (1-13), human
Catalog No.:BCC1013
CAS No.:100111-07-7
- Chloramultilide D
Catalog No.:BCN7102
CAS No.:1000995-49-2
- Chloramultilide C
Catalog No.:BCN6618
CAS No.:1000995-48-1
- Chloramultilide B
Catalog No.:BCN6613
CAS No.:1000995-47-0
- H-Lys(Tfa)-OH
Catalog No.:BCC2985
CAS No.:10009-20-8
- Piscidinol A
Catalog No.:BCN5818
CAS No.:100198-09-2
- 2,3-dihydroxy-3-(4-hydroxyphenyl)propanoic acid
Catalog No.:BCN1641
CAS No.:100201-57-8
- 5,5'-Dimethoxysecoisolariciresinol
Catalog No.:BCN7941
CAS No.:1002106-91-3
- Camstatin
Catalog No.:BCC5690
CAS No.:1002295-95-5
- AMG-208
Catalog No.:BCC1054
CAS No.:1002304-34-8
- Picrasidine I
Catalog No.:BCN5819
CAS No.:100234-59-1
- Picrasidine J
Catalog No.:BCN5820
CAS No.:100234-62-6
- TZ9
Catalog No.:BCC5547
CAS No.:1002789-86-7
- Irinotecan hydrochloride
Catalog No.:BCN2949
CAS No.:100286-90-6
- Apiopaeonoside
Catalog No.:BCN2801
CAS No.:100291-86-9
- Pemirolast potassium
Catalog No.:BCC4532
CAS No.:100299-08-9
- L(+)-Rhamnose monohydrate
Catalog No.:BCN8368
CAS No.:10030-85-0
Discovery of 1-[3-(1-methyl-1H-pyrazol-4-yl)-5-oxo-5H-benzo[4,5]cyclohepta[1,2-b]pyridin-7-yl] -N-(pyridin-2-ylmethyl)methanesulfonamide (MK-8033): A Specific c-Met/Ron dual kinase inhibitor with preferential affinity for the activated state of c-Met.[Pubmed:23379595]
J Med Chem. 2013 Mar 28;56(6):2294-310.
This report documents the first example of a specific inhibitor of protein kinases with preferential binding to the activated kinase conformation: 5H-benzo[4,5]cyclohepta[1,2-b]pyridin-5-one 11r (MK-8033), a dual c-Met/Ron inhibitor under investigation as a treatment for cancer. The design of 11r was based on the desire to reduce time-dependent inhibition of CYP3A4 (TDI) by members of this structural class. A novel two-step protocol for the synthesis of benzylic sulfonamides was developed to access 11r and analogues. We provide a rationale for the observed selectivity based on X-ray crystallographic evidence and discuss selectivity trends with additional examples. Importantly, 11r provides full inhibition of tumor growth in a c-Met amplified (GTL-16) subcutaneous tumor xenograft model and may have an advantage over inactive form kinase inhibitors due to equal potency against a panel of oncogenic activating mutations of c-Met in contrast to c-Met inhibitors without preferential binding to the active kinase conformation.


