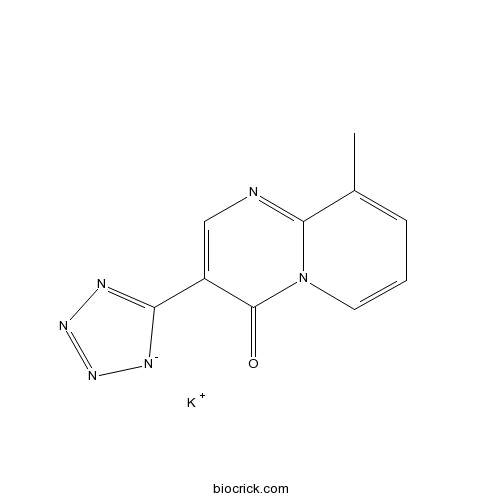
Pemirolast potassiumCAS# 100299-08-9 |
- Pralatrexate
Catalog No.:BCC2304
CAS No.:146464-95-1
- Pyrimethamine
Catalog No.:BCC2307
CAS No.:58-14-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 100299-08-9 | SDF | Download SDF |
| PubChem ID | 443866 | Appearance | Powder |
| Formula | C10H7KN6O | M.Wt | 266.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 200 mg/mL (751.03 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | potassium;9-methyl-3-(1,2,3-triaza-4-azanidacyclopenta-2,5-dien-5-yl)pyrido[1,2-a]pyrimidin-4-one | ||
| SMILES | CC1=CC=CN2C1=NC=C(C2=O)C3=NN=N[N-]3.[K+] | ||
| Standard InChIKey | NMMVKSMGBDRONO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H7N6O.K/c1-6-3-2-4-16-9(6)11-5-7(10(16)17)8-12-14-15-13-8;/h2-5H,1H3;/q-1;+1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Pemirolast potassium Dilution Calculator

Pemirolast potassium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7552 mL | 18.7758 mL | 37.5516 mL | 75.1033 mL | 93.8791 mL |
| 5 mM | 0.751 mL | 3.7552 mL | 7.5103 mL | 15.0207 mL | 18.7758 mL |
| 10 mM | 0.3755 mL | 1.8776 mL | 3.7552 mL | 7.5103 mL | 9.3879 mL |
| 50 mM | 0.0751 mL | 0.3755 mL | 0.751 mL | 1.5021 mL | 1.8776 mL |
| 100 mM | 0.0376 mL | 0.1878 mL | 0.3755 mL | 0.751 mL | 0.9388 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pemirolast potassium (BMY 26517) is a histamine H1 antagonist and mast cell stabilizer that acts as an antiallergic agent.
- Apiopaeonoside
Catalog No.:BCN2801
CAS No.:100291-86-9
- Irinotecan hydrochloride
Catalog No.:BCN2949
CAS No.:100286-90-6
- TZ9
Catalog No.:BCC5547
CAS No.:1002789-86-7
- Picrasidine J
Catalog No.:BCN5820
CAS No.:100234-62-6
- Picrasidine I
Catalog No.:BCN5819
CAS No.:100234-59-1
- AMG-208
Catalog No.:BCC1054
CAS No.:1002304-34-8
- Camstatin
Catalog No.:BCC5690
CAS No.:1002295-95-5
- 5,5'-Dimethoxysecoisolariciresinol
Catalog No.:BCN7941
CAS No.:1002106-91-3
- 2,3-dihydroxy-3-(4-hydroxyphenyl)propanoic acid
Catalog No.:BCN1641
CAS No.:100201-57-8
- Piscidinol A
Catalog No.:BCN5818
CAS No.:100198-09-2
- MK-8033
Catalog No.:BCC1768
CAS No.:1001917-37-8
- INH6
Catalog No.:BCC5455
CAS No.:1001753-24-7
- L(+)-Rhamnose monohydrate
Catalog No.:BCN8368
CAS No.:10030-85-0
- Curcumenone
Catalog No.:BCN3008
CAS No.:100347-96-4
- Calcium chloride dihydrate
Catalog No.:BCC7582
CAS No.:10035-04-8
- GSK 1562590 hydrochloride
Catalog No.:BCC8010
CAS No.:1003878-07-6
- Sodium Picosulfate
Catalog No.:BCC4845
CAS No.:10040-45-6
- Danshinspiroketallactone
Catalog No.:BCN3754
CAS No.:100414-80-0
- Lercanidipine
Catalog No.:BCC5239
CAS No.:100427-26-7
- Boric acid
Catalog No.:BCC7592
CAS No.:10043-35-3
- Cobicistat (GS-9350)
Catalog No.:BCC2271
CAS No.:1004316-88-4
- Dihydroresveratrol 3-O-glucoside
Catalog No.:BCN5821
CAS No.:100432-87-9
- 1-EBIO
Catalog No.:BCC6904
CAS No.:10045-45-1
- FFN 511
Catalog No.:BCC7799
CAS No.:1004548-96-2
Preventive effect of an antiallergic drug, pemirolast potassium, on restenosis after stent placement: quantitative coronary angiography and intravascular ultrasound studies.[Pubmed:12892037]
J Cardiol. 2003 Jul;42(1):13-22.
OBJECTIVES: The preventive effect of pemirolast against restenosis after coronary stent placement was evaluated. METHODS: Eighty-four patients with 89 de novo lesions who underwent successful coronary stenting were assigned to the pemirolast group(40 patients, 45 lesions) and the control group(44 patients, 44 lesions). Administration of pemirolast(20 mg/day) was initiated from the next morning after stenting and continued for 6 months of follow-up. Quantitative coronary angiography was performed immediately after stenting and at follow-up. Angiographic restenosis was defined as diameter stenosis > or = 50% at follow-up. Intravascular ultrasound study conducted at follow-up angiography was used to measure vessel cross-sectional area(CSA), stent CSA, lumen CSA, neointima CSA(stent CSA--lumen CSA), and percentage neointima CSA(neointima CSA/stent CSA x 100%) at the minimal lumen site. RESULTS: There were no significant differences in baseline characteristics between the two groups. Restenosis rate was significantly lower in the pemirolast group than in the control group(15.0% vs 34.1% of patients, 13.3% vs 34.1% of lesions, p < 0.05, respectively). The intravascular ultrasound study at follow-up(36 lesions in the pemirolast group, 33 in the control group) found no significant differences in vessel CSA and stent CSA between the two groups(17.3 +/- 2.2 vs 16.8 +/- 2.4 mm2, 8.6 +/- 1.9 vs 8.4 +/- 1.7 mm2, respectively). However, lumen CSA was significantly larger in the pemirolast group than in the control group(5.5 +/- 1.3 vs 4.4 +/- 1.1 mm2, p < 0.05). Moreover, neointima CSA and percentage neointima CSA were significantly smaller in the pemirolast group(3.1 +/- 1.1 vs 4.0 +/- 1.2 mm2, p < 0.05 and 36.2 +/- 15.9% vs 47.4 +/- 15.6%, p < 0.01). CONCLUSIONS: Pemirolast has a preventive effect against restenosis after stent placement, possibly by inhibiting neointimal hyperplasia.
A comparative trial of the safety and efficacy of 0.1 percent pemirolast potassium ophthalmic solution dosed twice or four times a day in patients with seasonal allergic conjunctivitis.[Pubmed:15117570]
J Ocul Pharmacol Ther. 2004 Apr;20(2):139-50.
PURPOSE: To compare efficacy and safety between twice-daily and four-times-daily regimens of pemirolast 0.1% in allergic conjunctivitis patients. METHODS: This investigator-masked trial recruited 169 patients, with a positive skin prick test, +2 itching and hyperemia. Patients were randomized to two times daily (t.i.d.) or four times daily (q.i.d.) treatment during allergy season. Evaluation was at 0, 2 and 4 weeks, with itching and hyperemia at week 4 as the primary endpoints. Analysis used last observation carried forward (LOCF) and analysis of variance (ANOVA) for efficacy, factoring treatment and center variations. The basis of the statistical evaluation was to confirm parity between two treatments, via noninferiority hypothesis testing. A 95% confidence interval (CI) with an upper limit of < or = 0.5 was set to assess non-inferiority or to conclude if schedules were statistically similar. RESULTS: B.i.d. and q.i.d. baselines were similar, respectively, for itching (2.6 and 2.8) and hyperemia (2.3 and 2.2). Week 4 itching was statistically non-inferior between treatments (1 b.i.d. versus 0.8 q.i.d.), with a mean treatment difference of 0.17 (-0.13, 0.47, Delta < or = 0.5). Week 4 hyperemia was comparable (1.2 for b.i.d. versus 1.0 for q.i.d). Week 2 scores and mean change from baseline (weeks 2 and 4), patient diary data, and investigator assessments were comparable. Both regimens were well tolerated with no differences in adverse events were observed. CONCLUSIONS: B.i.d. dosing was statistically non-inferior to q.i.d. dosing with respect to itching and hyperemia. Both regimens were similarly well tolerated in allergic conjunctivitis patients.
Comparison of 0.1% bromfenac sodium and 0.1% pemirolast potassium for the treatment of allergic conjunctivitis.[Pubmed:15592786]
Jpn J Ophthalmol. 2004 Nov-Dec;48(6):587-90.
PURPOSE: We compared the efficacy of a new nonsteroidal antiinflammatory drug (NSAID) eye drop, 0.1% bromfenac sodium (Bromfenac), with that of an antiallergic agent, 0.1% Pemirolast potassium (Pemirolast), in the treatment of seasonal allergic conjunctivitis in Japanese patients. METHODS: Twenty-two subjects with seasonal allergic conjunctivitis were enrolled in the study. One eye was treated with Bromfenac eye drops and the contralateral eye was treated with Pemirolast eye drops for 1 week. Subjective ocular symptoms and objective ocular signs evaluated by slit-lamp examination were scored and recorded before and after treatment. RESULTS: Both drugs significantly decreased ocular signs after 1 week, but not symptoms. No significant differences in subjective symptoms or objective signs were observed between the two drugs. Ten patients (45.5%) selected Bromfenac as more effective, nine patients (40.9%) selected Pemirolast, and three patients found no difference in efficacy between the two drugs. CONCLUSION: Bromfenac sodium is as safe and effective for the treatment of allergic conjunctivitis as Pemirolast potassium.
Suppressive activity of pemirolast potassium, an antiallergic drug, on glomerulonephritis. Studies in glomerulonephritis model rats and in patients with chronic glomerulonephritis concurrently affected by allergic rhinitis.[Pubmed:18368946]
Arzneimittelforschung. 2008;58(1):18-23.
BACKGROUND: It is still difficult to manage chronic glomerulonephritis with corticosteroids because of safety concerns, especially for patients with mild symptoms and infants. Therefore, an alternative approach is greatly required. Pemirolast potassium (CAS 100299-08-9) is an antiallergic drug with high safety. METHODS: Two glomerulonephritis rat models were prepared to examine the pharmacological actions of Pemirolast potassium: one reversible model prepared with the anti-Thy-1 antibody, and another irreversible model by unilateral nephrectomy and with the anti-Thy-1 antibody. Pemirolast potassium was administered to 10 Japanese chronic glomerulonephritis patients concurrently affected by allergic rhinitis in order to examine its efficacy for mild proteinuria. RESULTS: Pemirolast potassium 1 and 10 mg/kg markedly inhibited proteinuria in the reversible model. In the irreversible model, Pemirolast potassium 3 mg/kg showed a significant decrease in the incidence of glomerulosclerosis. In chronic glomerulonephritis patients, Pemirolast potassium, 10 mg twice daily, for 6 months, significantly reduced the severity of proteinuria. CONCLUSION: Our research suggested the efficacy of Pemirolast potassium in glomerulonephritis. A well-controlled study is considered necessary to validate Pemirolast potassium as a therapeutic drug for glomerulonephritis.


