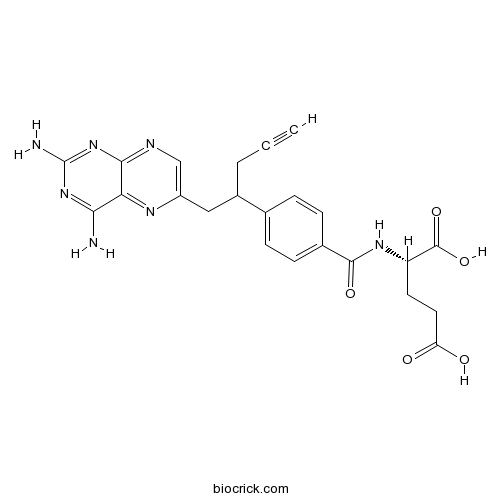
PralatrexateAntifolate,a folate analog CAS# 146464-95-1 |
- Pemetrexed
Catalog No.:BCC9115
CAS No.:137281-23-3
- Pemetrexed disodium hemipenta hydrate
Catalog No.:BCC1844
CAS No.:357166-30-4
- Pyrimethamine
Catalog No.:BCC2307
CAS No.:58-14-0
- Methotrexate
Catalog No.:BCC2301
CAS No.:59-05-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 146464-95-1 | SDF | Download SDF |
| PubChem ID | 148121 | Appearance | Powder |
| Formula | C23H23N7O5 | M.Wt | 477.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (104.72 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2S)-2-[[4-[1-(2,4-diaminopteridin-6-yl)pent-4-yn-2-yl]benzoyl]amino]pentanedioic acid | ||
| SMILES | C#CCC(CC1=CN=C2C(=N1)C(=NC(=N2)N)N)C3=CC=C(C=C3)C(=O)NC(CCC(=O)O)C(=O)O | ||
| Standard InChIKey | OGSBUKJUDHAQEA-WMCAAGNKSA-N | ||
| Standard InChI | InChI=1S/C23H23N7O5/c1-2-3-14(10-15-11-26-20-18(27-15)19(24)29-23(25)30-20)12-4-6-13(7-5-12)21(33)28-16(22(34)35)8-9-17(31)32/h1,4-7,11,14,16H,3,8-10H2,(H,28,33)(H,31,32)(H,34,35)(H4,24,25,26,29,30)/t14?,16-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pralatrexate(Folotyn) is an antifolate, and structurally a folate analog. Its IC50 is < 300 nM in some cell lines.
IC50 Value: < 300 nM
Target: Antifolate
Pralatrexate is an antifolate (a folate analogue metabolic inhibitor) designed to accumulate preferentially in cancer cells. Based on preclinical studies, researchers believe that Pralatrexate selectively enters cells expressing reduced folate carrier type 1 (RFC-1), a protein that is overexpressed on certain cancer cells compared to normal cells. References: | |||||

Pralatrexate Dilution Calculator

Pralatrexate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0944 mL | 10.4719 mL | 20.9437 mL | 41.8874 mL | 52.3593 mL |
| 5 mM | 0.4189 mL | 2.0944 mL | 4.1887 mL | 8.3775 mL | 10.4719 mL |
| 10 mM | 0.2094 mL | 1.0472 mL | 2.0944 mL | 4.1887 mL | 5.2359 mL |
| 50 mM | 0.0419 mL | 0.2094 mL | 0.4189 mL | 0.8377 mL | 1.0472 mL |
| 100 mM | 0.0209 mL | 0.1047 mL | 0.2094 mL | 0.4189 mL | 0.5236 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pralatrexate is an inhibitor of DHFR with Ki value of 45 nM [1].
Dihydrofolate reductase (DHFR) is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, a methyl group shuttle required for the synthesis of purines, thymidylic acid, and certain amino acids.
Pralatrexate is a DHFR inhibitor with high affinity for folylpolyglutamate synthetase (FPGS) and reduced folate carrier 1 (RFC-1), resulting in extensive internalization and accumulation in tumour cells. In 15 human cancer cell lines, pralatrexate showed antiproliferative effects with IC50 < 0.1 μM in PC3, SCC61, DU145, HT29, HOP62, SQ20B, HOP92, HEP2 and IGROV1 cells. While it showed antiproliferative effects with IC50≥ 9 μM in Colo205, HCC2998, MCF7, HCT116, OVCAR3 and MDA-MB-435 cells [2].
In MV522 human non-small cell lung cancer (NSCLC) xenograft, pralatrexate showed increased antitumor activity. In the 2 mg/kg pralatrexate-treated group, the 38% tumor growth inhibition (TGI) was observed. In NCI-H460 NSCLC xenograft, pralatrexate showed antitumor activity in a dose-dependent way. TGI of 1 mg/kg and 2 mg/kg pralatrexate-treated groups was 34% and 52%, respectively. In the two xenografts, pralatrexate resulted in weight loss, which suggested its toxicity [1].
References:
[1]. Izbicka E, Diaz A, Streeper R, et al. Distinct mechanistic activity profile of pralatrexate in comparison to other antifolates in in vitro and in vivo models of human cancers. Cancer Chemother Pharmacol, 2009, 64(5): 993-999.
[2]. Serova M, Bieche I, Sablin MP, et al. Single agent and combination studies of pralatrexate and molecular correlates of sensitivity. Br J Cancer, 2011, 104(2): 272-280.
- Camaric acid
Catalog No.:BCN1650
CAS No.:146450-83-1
- Flavopiridol
Catalog No.:BCC1577
CAS No.:146426-40-6
- Lactose
Catalog No.:BCN8387
CAS No.:14641-93-1
- Desmethylrocaglamide
Catalog No.:BCN7735
CAS No.:146408-78-8
- SR 48692
Catalog No.:BCC7763
CAS No.:146362-70-1
- N-Methyllidocaine iodide
Catalog No.:BCC6905
CAS No.:1462-71-1
- Chlorajapolide F
Catalog No.:BCN6425
CAS No.:1461760-59-7
- R-(-)-Deprenyl hydrochloride
Catalog No.:BCC5196
CAS No.:14611-52-0
- Z-Arg(Z)2-OH
Catalog No.:BCC3574
CAS No.:14611-34-8
- Pulchinenoside E1
Catalog No.:BCN8185
CAS No.:146100-02-9
- Dihydromarein
Catalog No.:BCN8406
CAS No.:
- MSDC-0160
Catalog No.:BCC5343
CAS No.:146062-49-9
- 1-Methylpsilocin
Catalog No.:BCC7536
CAS No.:1465-16-3
- Complanatoside A
Catalog No.:BCN6282
CAS No.:146501-37-3
- WR 1065
Catalog No.:BCC2417
CAS No.:14653-77-1
- Tyrphostin AG 1296
Catalog No.:BCC1195
CAS No.:146535-11-7
- Fmoc-Gly(allyl)-OH
Catalog No.:BCC3156
CAS No.:146549-21-5
- 2-Cyclopropyl-3-[(diphenylphosphinyl)methyl]-4-(4-fluorophenyl)quinoline
Catalog No.:BCC8572
CAS No.:146578-99-6
- 2,6-Dimethoxybenzoic acid
Catalog No.:BCN1651
CAS No.:1466-76-8
- Dantrolene, sodium salt
Catalog No.:BCC6673
CAS No.:14663-23-1
- H-Trp(Boc)-OH
Catalog No.:BCC3115
CAS No.:146645-63-8
- SR 2640 hydrochloride
Catalog No.:BCC7180
CAS No.:146662-42-2
- (RS)-MCPG
Catalog No.:BCC6610
CAS No.:146669-29-6
- SR 11237
Catalog No.:BCC7681
CAS No.:146670-40-8
Phase 1 and 2 study of carboplatin and pralatrexate in patients with recurrent, platinum-sensitive ovarian, fallopian tube, or primary peritoneal cancer.[Pubmed:27421044]
Cancer. 2016 Nov 15;122(21):3297-3306.
BACKGROUND: The objective of this phase 1 and 2 trial was to identify the appropriate dose of combined carboplatin and Pralatrexate for patients with recurrent, platinum-sensitive ovarian, fallopian tube, and primary peritoneal cancer. METHODS: In phase 1, patients received carboplatin (at an area under the curve of 5) and increasing doses of Pralatrexate until the maximum-tolerated dose (MTD) of Pralatrexate was achieved. The primary endpoint was the response rate. Additional endpoints were safety, response duration, progression-free survival, overall survival, and pharmacokinetics. RESULTS: Thirty patients were enrolled in phase 1, and 20 were enrolled in phase 2. Of all 50 patients, 49 completed the study. The mean patient age was 59 years, and patients completed a median of 6 cycles. The MTD for Pralatrexate was 105 mg/m(2) . The clinical benefit rate (complete responses plus partial responses plus stable disease) was 86%. Of 26 patients who received the MTD, 12 had a partial response, 11 had stable disease, and 2 had disease progression. The progression-free survival rate at 3 and 6 months was 87% and 79%, respectively; and the overall survival rate was 98% at 6 and 12 months and 66% at 24 months. Of 30 patients, 18 (60%) in phase 1 experienced an adverse event of any grade; and, of those, 4 patients (13%) had a grade 3 or greater adverse event. In phase 2, 12 patients (60%) had an adverse event of any grade, and 4 (20%) had grade 3 or greater toxicity. There was a significant reduction in the total body clearance of Pralatrexate when it was received concurrently with carboplatin. CONCLUSIONS: Most patients responded to carboplatin-Pralatrexate combination. This regimen is well tolerated and effective in this patient population. Cancer 2016;122:3297-3306. (c) 2016 American Cancer Society.
Phase 1 study evaluating the safety and pharmacokinetics of pralatrexate in relapsed/refractory advanced solid tumors and lymphoma patients with mild, moderate, and severe renal impairment.[Pubmed:27638045]
Cancer Chemother Pharmacol. 2016 Nov;78(5):929-939.
PURPOSE: Pralatrexate is a folate analogue indicated for the treatment of relapsed or refractory peripheral T-cell lymphoma. It has not been formally tested in patients with renal impairment. This study evaluated the pharmacokinetic (PK) profile of Pralatrexate in patients with renal impairment and with relapsed/refractory advanced solid tumors and lymphoma. METHODS: This was an open-label, nonrandomized, phase 1 study. Eligible patients received Pralatrexate administered as an IV push over 3-5 min once weekly for 6 weeks in 7-week cycles until progressive disease or intolerable toxicity. Four cohorts of 6 patients were planned for a total of 24 patients. Patients with normal renal function (Cohort A), mild (Cohort B), and moderate renal impairment (Cohort C) received 30 mg/m(2) Pralatrexate once weekly for 6 weeks in 7-week cycles, and patients with severe renal impairment (Cohort D) were to be administered 20 mg/m(2) once weekly for 6 weeks. Plasma and urine samples were collected at pre-specified time points to determine the PK profile of Pralatrexate in each treatment cohort. Patients were followed for safety and tolerability. RESULTS: A total of 29 patients were enrolled and 27 patients (14 male) received at least 1 dose of Pralatrexate. Because of a qualifying toxicity in Cohort C, the starting dose for Cohort D was reduced to 15 mg/m(2). Chronic renal impairment led to a decrease in renal clearance of the Pralatrexate diastereomers, PDX-10a and PDX-10b, but systemic exposure to these diastereomers was not dramatically affected by renal impairment. Pralatrexate exposure in Cohort D (15 mg/m(2)) was similar to the exposure in other cohorts (30 mg/m(2)). No apparent difference in toxicity between the four treatment cohorts was observed, except for an increase in cytopenias in patients with severe renal impairment. CONCLUSION: Pralatrexate exposure, at a dose of 30 mg/m(2), in patients with mild or moderate renal impairment was similar to the exposure in patients with normal renal function. For patients with severe renal impairment only, a Pralatrexate dose of 15 mg/m(2) is recommended.
Results from a Phase I/II Open-Label, Dose-Finding Study of Pralatrexate and Oral Bexarotene in Patients with Relapsed/Refractory Cutaneous T-cell Lymphoma.[Pubmed:28167509]
Clin Cancer Res. 2017 Jul 15;23(14):3552-3556.
Purpose: Pralatrexate is a folic acid analogue metabolic inhibitor similar to methotrexate, which has shown tolerability and efficacy with an overall response rate of 45% in a phase I dose deescalation study of patients with relapsed/refractory cutaneous T-cell lymphoma (CTCL).Experimental Design: The object of this phase I/II open-label, multicenter clinical trial was to determine the MTD and recommended dose of Pralatrexate plus oral bexarotene in 34 patients with relapsed/refractory CTCL who had failed prior systemic therapies. Pralatrexate was administered by intravenous push at 15 mg/m(2) given weekly 3 weeks out of 4 weeks with daily oral bexarotene (150 or 300 mg/m(2)), levothyroxine, atorvastatin, folate, and with B12 every 2 months.Results: At the MTD of 15 mg/m(2) bexarotene and 15 mg/m(2) Pralatrexate, the response rate was 60% [4 complete responses (CR), 14 partial responses (PR)], the maximum observed response duration was 28.9+ months, and duration of response for 4 CRs ranged from 9.0 to 28.3 months. The median progression-free survival was 12.8 months (0.5-29.9). Mucositis was the most common adverse event.Conclusions: The combination of Pralatrexate (15 mg/m(2)) and oral bexarotene (150 mg/m(2)) is active with high response rates and minimal toxicity for cutaneous T-cell lymphomas. Clin Cancer Res; 23(14); 3552-6. (c)2017 AACR.


