PyrimethamineDHFR inhibitor CAS# 58-14-0 |
- Pemetrexed
Catalog No.:BCC9115
CAS No.:137281-23-3
- Pralatrexate
Catalog No.:BCC2304
CAS No.:146464-95-1
- Pemetrexed disodium hemipenta hydrate
Catalog No.:BCC1844
CAS No.:357166-30-4
- Methotrexate
Catalog No.:BCC2301
CAS No.:59-05-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 58-14-0 | SDF | Download SDF |
| PubChem ID | 4993 | Appearance | Powder |
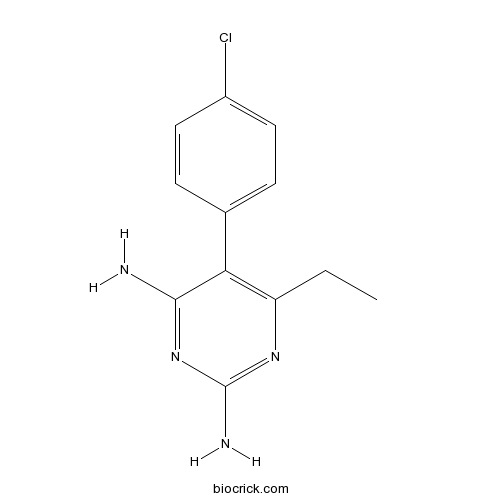
| Formula | C12H13ClN4 | M.Wt | 248.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Pirimecidan; Pirimetamin; RP 4753 | ||
| Solubility | DMSO : 25 mg/mL (100.52 mM; Need ultrasonic) | ||
| Chemical Name | 5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine | ||
| SMILES | CCC1=C(C(=NC(=N1)N)N)C2=CC=C(C=C2)Cl | ||
| Standard InChIKey | WKSAUQYGYAYLPV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of multidrug and toxin extrusion (MATE) transporters (Ki values are 46 and 77 nM for human MATE2-K-HEK293 and MATE1-HEK293 cells respectively). Also inhibits dihydrofolate reductase (DHFR) and STAT3. Decreases proliferation of human autosomal dominant polycystic kidney disease cells. |

Pyrimethamine Dilution Calculator

Pyrimethamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0207 mL | 20.1037 mL | 40.2075 mL | 80.4149 mL | 100.5187 mL |
| 5 mM | 0.8041 mL | 4.0207 mL | 8.0415 mL | 16.083 mL | 20.1037 mL |
| 10 mM | 0.4021 mL | 2.0104 mL | 4.0207 mL | 8.0415 mL | 10.0519 mL |
| 50 mM | 0.0804 mL | 0.4021 mL | 0.8041 mL | 1.6083 mL | 2.0104 mL |
| 100 mM | 0.0402 mL | 0.201 mL | 0.4021 mL | 0.8041 mL | 1.0052 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pyrimethamine is a dihydrofolate reductase(DHFR) inhibitor with an IC50 of 15.4 nM.
- Caffeine
Catalog No.:BCN5807
CAS No.:58-08-2
- Bax inhibitor peptide P5
Catalog No.:BCC2393
CAS No.:579492-83-4
- Bax inhibitor peptide V5
Catalog No.:BCC2394
CAS No.:579492-81-2
- Officinalisinin I
Catalog No.:BCN2825
CAS No.:57944-18-0
- L(+)-Asparagine Monohydrate
Catalog No.:BCC8332
CAS No.:5794-13-8
- Z-Cys(Z)-OH
Catalog No.:BCC2784
CAS No.:57912-35-3
- Corynoxidine
Catalog No.:BCN6798
CAS No.:57906-85-1
- 19-Nor-4-hydroxyabieta-8,11,13-trien-7-one
Catalog No.:BCN1411
CAS No.:57906-31-7
- o-Anisic acid
Catalog No.:BCC9108
CAS No.:579-75-9
- Lobelanine
Catalog No.:BCN2156
CAS No.:579-21-5
- Oligomycin A
Catalog No.:BCC2530
CAS No.:579-13-5
- Myrianthic acid 3,23-acetonide
Catalog No.:BCN7517
CAS No.:578710-52-8
- Aminophenazone
Catalog No.:BCC8815
CAS No.:58-15-1
- Methyltestosterone
Catalog No.:BCC9045
CAS No.:58-18-4
- Testosterone cypionate
Catalog No.:BCC9167
CAS No.:58-20-8
- Testosterone
Catalog No.:BCN2193
CAS No.:58-22-0
- Menadione
Catalog No.:BCN8351
CAS No.:58-27-5
- Desipramine hydrochloride
Catalog No.:BCC7553
CAS No.:58-28-6
- Promethazine HCl
Catalog No.:BCC5480
CAS No.:58-33-3
- Prochlorperazine
Catalog No.:BCC3846
CAS No.:58-38-8
- Tetrabenazine
Catalog No.:BCC5277
CAS No.:58-46-8
- Theophylline
Catalog No.:BCN1258
CAS No.:58-55-9
- Pyridoxine HCl
Catalog No.:BCC4835
CAS No.:58-56-0
- Puromycin dihydrochloride
Catalog No.:BCC7860
CAS No.:58-58-2
Estimated impact on birth weight of scaling up intermittent preventive treatment of malaria in pregnancy given sulphadoxine-pyrimethamine resistance in Africa: A mathematical model.[Pubmed:28245259]
PLoS Med. 2017 Feb 28;14(2):e1002243.
BACKGROUND: Malaria transmission has declined substantially in the 21st century, but pregnant women in areas of sustained transmission still require protection to prevent the adverse pregnancy and birth outcomes associated with malaria in pregnancy (MiP). A recent call to action has been issued to address the continuing low coverage of intermittent preventive treatment of malaria in pregnancy (IPTp). This call has, however, been questioned by some, in part due to concerns about resistance to sulphadoxine-Pyrimethamine (SP), the only drug currently recommended for IPTp. METHODS AND FINDINGS: Using an existing mathematical model of MiP, we combined estimates of the changing endemicity of malaria across Africa with maps of SP resistance mutations and current coverage of antenatal access and IPTp with SP (IPTp-SP) across Africa. Using estimates of the relationship between SP resistance mutations and the parasitological efficacy of SP during pregnancy, we estimated the varying impact of IPTp-SP across Africa and the incremental value of enhancing IPTp-SP uptake to match current antenatal care (ANC) coverage. The risks of MiP and malaria-attributable low birthweight (mLBW) in unprotected pregnancies (i.e., those not using insecticide-treated nets [ITNs]) leading to live births fell by 37% (33%-41% 95% credible interval [crI]) and 31% (27%-34% 95% crI), respectively, from 2000 to 2015 across endemic areas in sub-Saharan Africa. However, these gains are fragile, and coverage is far from optimal. In 2015, 9.5 million (8.3 million-10.4 million 95% crI) of 30.6 million pregnancies in these areas would still have been infected with Plasmodium falciparum without intervention, leading to 750,000 (390,000-1.1 million 95% crI) mLBW deliveries. In all, 6.6 million (5.6 million-7.3 million 95% crI) of these 9.5 million (69.3%) pregnancies at risk of infection (and 53.4% [16.3 million/30.6 million] of all pregnancies) occurred in settings with near-perfect SP curative efficacy (>99%) based on the most recent estimates of resistance. Forty-four percent of these pregnancies (23% of all pregnancies) were not receiving any IPTp-SP despite making >/=3 ANC visits, representing 160,000 (94,000-236,000 95% crI) preventable low birthweight (LBW) deliveries. Only 4% (1.4 million) of pregnancies occurred in settings with >10% prevalence of the sextuple haplotype associated with compromised SP effectiveness. Forty-two percent of all pregnancies occurred in settings where the quintuple dhfr/dhps haplotype had become established but where in vivo efficacy data suggest SP maintains the majority of its effectiveness in clearing infections. Not accounting for protection from the use of ITNs during pregnancy, expanding IPTp-SP to all women with >/=3 ANC visits in Africa could prevent an additional 215,000 (128,000-318,000 95% crI) LBW deliveries. In 26 countries with sufficient recent data to estimate ITN impact (population-based ITN usage data that can be stratified by gravidity), we estimate that, due primarily to low ITN use by primigravidae, only 16.5% of the potential LBW births prevented by scaling up IPTp-SP would in fact have already have been prevented through ITN use. Our analysis also highlights the difficulties associated with estimating the relationship between the effectiveness of interventions against parasitological endpoints such as placental infection at delivery and health outcomes including birthweight, which is also determined by a wide range of unrelated factors. We also did not capture other aspects of malaria burden such as clinical malaria, maternal and neonatal anaemia, and miscarriage, all of which increase the overall importance of effective preventative strategies but have their own relationship with transmission intensity, parity, and SP resistance. CONCLUSIONS: Despite recent declines in malaria transmission in Africa, the burden of MiP in the absence of adequate prevention remains substantial. Even accounting for SP resistance, extending IPTp-SP to all women attending ANC, as well as long-lasting insecticidal net distribution targeted towards first-time mothers, would have a sizeable impact upon maternal and infant health in almost all malaria-endemic settings in sub-Saharan Africa.
Sulfadoxine-Pyrimethamine Exhibits Dose-Response Protection Against Adverse Birth Outcomes Related to Malaria and Sexually Transmitted and Reproductive Tract Infections.[Pubmed:28329383]
Clin Infect Dis. 2017 Apr 15;64(8):1043-1051.
Background: We conducted a prospective cohort study in Zambia among pregnant women who received intermittent preventive treatment using sulfadoxine-Pyrimethamine (IPTp-SP). Methods: We calculated the odds ratios (ORs) of adverse birth outcomes by IPTp-SP exposure, 0-1 dose (n = 126) vs >/=2 doses (n = 590) and >/=2 doses (n = 310) vs >/=3 doses (n = 280) in 7 categories of malaria infection and sexually transmitted and reproductive tract infections (STIs/RTIs). Results: We found no significant differences in baseline prevalence of infection across IPTp-SP exposure groups. However, among women given 2 doses compared to 0-1 dose, the odds of any adverse birth outcome were reduced 45% (OR, 0.55; 95% confidence interval [CI], 0.36, 0.86) and 13% further with >/=3 doses (OR, 0.43; 95% CI, 0.27, 0.68). Two or more doses compared to 0-1 dose reduced preterm delivery by 58% (OR, 0.42; 95% CI, 0.27, 0.67) and 21% further with >/=3 doses (OR, 0.21; 95% CI, 0.13, 0.35). Women with malaria at enrollment who received >/=2 doses vs 0-1 had 76% lower odds of any adverse birth outcome (OR, 0.24; 95% 0.09, 0.66), and Neisseria gonorrhoeae and/or Chlamydia trachomatis had 92% lower odds of any adverse birth outcome (OR, 0.08; 95% CI, 0.01, 0.64). Women with neither a malaria infection nor STIs/RTIs who received >/=2 doses had 73% fewer adverse birth outcomes (OR, 0.27; 95% CI, 0.11, 0.68). Conclusions: IPTp-SP appears to protect against malaria, STIs/RTIs, and other unspecified causes of adverse birth outcome.
Changing the policy for intermittent preventive treatment with sulfadoxine-pyrimethamine during pregnancy in Malawi.[Pubmed:28219435]
Malar J. 2017 Feb 20;16(1):84.
BACKGROUND: The growing resistance of Plasmodium falciparum to sulfadoxine-Pyrimethamine (SP) treatment for uncomplicated malaria led to a recommendation by the World Health Organization for the use of artemisinin-based combination therapy. Inevitably, concerns were also raised surrounding the use of SP for intermittent prevention treatment of malaria during pregnancy (IPTp) amidst the lack of alternative drugs. Malawi was the first country to adopt intermittent prevention treatment with SP in 1993, and updated in 2013. This case study examines the policy updating process and the contribution of research and key stakeholders to this process. The findings support the development of a malaria research-to-policy framework in Malawi. METHODS: Documents and evidence published from 1993 to 2012 were systematically reviewed in addition to key informant interviews. RESULTS: The online search identified 170 potential publications, of which eight from Malawi met the inclusion criteria. Two published studies from Malawi were instrumental in the WHO policy recommendation which in turn led to the updating of national policies. The updated policy indicates that more than two SP doses, as informed by research, overcome the challenges of the first policy of two SP doses only because of ineffectiveness by P. falciparum resistance and the global lack of replacement drugs to SP for IPTp. CONCLUSION: International WHO recommendations facilitated a smooth policy change driven by motivated local leadership with technical and financial support from development partners. Policy development and implementation should include key stakeholders and use local malaria research in a research-to-policy framework.
Optimal Antimalarial Dose Regimens for Sulfadoxine-Pyrimethamine with or without Azithromycin in Pregnancy Based on Population Pharmacokinetic Modeling.[Pubmed:28242669]
Antimicrob Agents Chemother. 2017 Apr 24;61(5). pii: AAC.02291-16.
Optimal dosing of sulfadoxine-Pyrimethamine (SP) as intermittent preventive treatment in pregnancy remains to be established, particularly when coadministered with azithromycin (AZI). To further characterize SP pharmacokinetics in pregnancy, plasma concentration-time data from 45 nonpregnant and 45 pregnant women treated with SP-AZI (n = 15 in each group) and SP-chloroquine (n = 30 in each group) were analyzed. Population nonlinear mixed-effect pharmacokinetic models were developed for Pyrimethamine (PYR), sulfadoxine (SDOX), and N-acetylsulfadoxine (the SDOX metabolite NASDOX), and potential covariates were included. Pregnancy increased the relative clearance (CL/F) of PYR, SDOX, and NASDOX by 48, 29, and 70%, respectively, as well as the relative volumes of distribution (V/F) of PYR (46 and 99%) and NASDOX (46%). Coadministration of AZI resulted in a greater increase in PYR CL/F (80%) and also increased NASDOX V/F by 76%. Apparent differences between these results and those of published studies of SP disposition may reflect key differences in study design, including the use of an early postpartum follow-up study rather than a nonpregnant comparator group. Simulations based on the final population model demonstrated that, compared to conventional single-dose SP in nonpregnant women, two such doses given 24 h apart should ensure that pregnant women have similar drug exposure, while three daily SP doses may be required if SP is given with AZI. The results of past and ongoing trials using recommended adult SP doses with or without AZI in pregnant women may need to be interpreted in light of these findings and consideration given to using increased doses in future trials.
Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways.[Pubmed:21821671]
Hum Mol Genet. 2011 Nov 1;20(21):4143-54.
Autosomal dominant polycystic kidney disease (ADPKD) is a commonly inherited disorder mostly caused by mutations in PKD1, encoding polycystin-1 (PC1). The disease is characterized by development and growth of epithelium-lined cyst in both kidneys, often leading to renal failure. There is no specific treatment for this disease. Here, we report a sustained activation of the transcription factor signal transducer and activator of transcription 3 (STAT3) in ischemic injured and uninjured Pkd1 knockout polycystic kidneys and in human ADPKD kidneys. Through a chemical library screen, we identified the anti-parasitic compound Pyrimethamine as an inhibitor of STAT3 function. Treatment with Pyrimethamine decreases cell proliferation in human ADPKD cells and blocks renal cyst formation in an adult and a neonatal PKD mouse model. Moreover, we demonstrated that a specific STAT3 inhibitor, S3I-201, reduces cyst formation and growth in a neonatal PKD mouse model. Our results suggest that PC1 acts as a negative regulator of STAT3 and that blocking STAT3 signaling with Pyrimethamine or similar drugs may be an attractive therapy for human ADPKD.
Potent and specific inhibition of mMate1-mediated efflux of type I organic cations in the liver and kidney by pyrimethamine.[Pubmed:20065018]
J Pharmacol Exp Ther. 2010 Apr;333(1):341-50.
This report describes a potent and selective inhibitor of multidrug and toxin extrusion (MATE) protein, Pyrimethamine (PYR), and examines its effect on the urinary and biliary excretion of typical Mate1 substrates in mice. In vitro inhibition studies demonstrated that PYR is a potent inhibitor of mouse (m)Mate1 (K(i) = 145 nM) among renal organic cation transporters mOctn1 and mOctn2 (K(i) > 30 microM), mOct1 (K(i) = 3.6 microM), and mOct2 (K(i) = 6.0 microM). PYR inhibited the uptake of metformin by kidney brush-border membrane vesicles (BBMVs) (K(i) = 41 nM) and canalicular membrane vesicles in the presence of outward gradient of H+. PYR treatment significantly increased the kidney-to-plasma ratio of tetraethylammonium, and both the liver- and kidney-to-plasma ratios of metformin in mice, whereas it did not affect their plasma concentrations and urinary excretion rates. Furthermore, the plasma lactate concentration, a biomarker for inhibition of gluconeogenesis by metformin, was significantly higher in the PYR-treated group than in the control group. These results not only suggest the importance of mMate1 in the efflux of organic cations into the urine and bile in mice but also the importance of canalicular efflux mediated by MATE proteins for the therapeutic efficacy of metformin. PYR is a potent inhibitor of human (h)MATE1 and hMATE2-K (K(i) = 77 and 46 nM, respectively) and H+ and organic cation exchanger in human kidney BBMVs (K(i) = 31 nM) in the presence of outward gradient of H+. Taken together, PYR can be used as a potent probe inhibitor of human MATE transporters.
Competitive inhibition of folate absorption by dihydrofolate reductase inhibitors, trimethoprim and pyrimethamine.[Pubmed:3630970]
Am J Clin Nutr. 1987 Sep;46(3):518-22.
Trimethoprim and Pyrimethamine, inhibitors of dihydrofolate reductase (DHFR), cause folate deficiency in some patients. We investigated impairment of intestinal folate absorption by these drugs. By use of the in vivo intestinal-loop methods in rats, absorption of [3H] folic acid was significantly decreased in the presence of either drug. Kinetic studies using the influx chamber method demonstrated a pattern of competitive inhibition of folate transport. [3H] folic acid absorption from jejunal loops was determined 3-16 h after IV administration of methotrexate; this treatment abolished DHFR activity in the small intestine. In rats pretreated with methotrexate, luminal disappearance and systemic absorption of folic acid were significantly enhanced with respect to controls. Trimethoprim and Pyrimethamine are weak competitive inhibitors of intestinal folate transport and folate absorption inhibition occurs at the site of membrane transport and appears to be unrelated to concurrent inhibition of DHFR activity in enterocytes.


