Bax inhibitor peptide V5Bax inhibitor CAS# 579492-81-2 |
- Bavisant dihydrochloride hydrate
Catalog No.:BCC1404
CAS No.:1103522-80-0
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- Mianserin HCl
Catalog No.:BCC1114
CAS No.:21535-47-7
- Loratadine
Catalog No.:BCC1262
CAS No.:79794-75-5
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 579492-81-2 | SDF | Download SDF |
| PubChem ID | 10129115 | Appearance | Powder |
| Formula | C27H50N6O6S | M.Wt | 586.79 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BIP-V5; BAX Inhibiting Peptide V5 | ||
| Solubility | DMSO : ≥ 30 mg/mL (51.13 mM) *"≥" means soluble, but saturation unknown. | ||
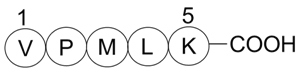
| Sequence | VPMLK | ||
| Chemical Name | (2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-1-[(2S)-2-amino-3-methylbutanoyl]pyrrolidine-2-carbonyl]amino]-4-methylsulfanylbutanoyl]amino]-4-methylpentanoyl]amino]hexanoic acid | ||
| SMILES | CC(C)CC(C(=O)NC(CCCCN)C(=O)O)NC(=O)C(CCSC)NC(=O)C1CCCN1C(=O)C(C(C)C)N | ||
| Standard InChIKey | NHMUTADCTDDWPV-YFNVTMOMSA-N | ||
| Standard InChI | InChI=1S/C27H50N6O6S/c1-16(2)15-20(24(35)31-19(27(38)39)9-6-7-12-28)32-23(34)18(11-14-40-5)30-25(36)21-10-8-13-33(21)26(37)22(29)17(3)4/h16-22H,6-15,28-29H2,1-5H3,(H,30,36)(H,31,35)(H,32,34)(H,38,39)/t18-,19-,20-,21-,22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cell-permeable synthetic peptide inhibitor of Bax conformational change and mitochondrial translocation. Designed based on the Bax-binding domain of human Ku70. Inhibits Bax-mediated apoptosis in vitro. Shown to inhibit anti-cancer drug-induced apoptosis in vitro. Negative control available. |

Bax inhibitor peptide V5 Dilution Calculator

Bax inhibitor peptide V5 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bax inhibitor peptide V5 (BIP V5) is a peptide inhibitor of Bax translocation to mitochondria [1].
Bax is a pro-apoptotic member of Bcl-2 family proteins and plays an important role in mitochondria-dependent apoptosis. Bax stays in the cytosol and transfers into mitochondria after apoptotic stimuli [1].
BIP V5 is a membrane-permeable peptide inhibitor of Bax translocation to mitochondria. In HeLa cells, BIP V5 protected cells from UVC- and STS-induced apoptosis. In U87-MG glioma cells, MCF-7 breast cancer cells and LNCaP prostate cancer cells, BIP V5 also inhibited apoptosis induced by anti-cancer drugs cisplatin, etoposide and doxorubicin. While BIP V5 did not suppress UVC- or STS-induced apoptosis in Bax-deficient cells (DU145), which suggested BIP V5 only suppressed Bax-mediated apoptosis. Also, BIP (V5) inhibited Bax translocation to mitochondria stimulated by UVC irradiation and STS treatment. The caspase activation and the release of cytochrome c from mitochondria triggered by apoptotic stimuli were also significantly inhibited by BIP V5. BIP V5 inhibited the interaction of Ku70 and endogenous Bax in a dose-dependent way [1].
In a mouse model, BIP V5 increased expression of anti-apoptotic proteins XIAP and Bcl-2 by more than 11- and 3-fold and reduced expression of apoptosis-inducing proteins Bax, Bad, and nuclear factor-κ B-p65 by 10, 30, and nearly 50%, respectively. Also, BIP V5 increased glucose-responsive insulin secretion [2].
References:
[1]. Sawada M, Hayes P, Matsuyama S. Cytoprotective membrane-permeable peptides designed from the Bax-binding domain of Ku70. Nat Cell Biol, 2003, 5(4): 352-357.
[2]. Rivas-Carrillo JD, Soto-Gutierrez A, Navarro-Alvarez N, et al. Cell-permeable pentapeptide V5 inhibits apoptosis and enhances insulin secretion, allowing experimental single-donor islet transplantation in mice. Diabetes, 2007, 56(5): 1259-1267.
- Officinalisinin I
Catalog No.:BCN2825
CAS No.:57944-18-0
- L(+)-Asparagine Monohydrate
Catalog No.:BCC8332
CAS No.:5794-13-8
- Z-Cys(Z)-OH
Catalog No.:BCC2784
CAS No.:57912-35-3
- Corynoxidine
Catalog No.:BCN6798
CAS No.:57906-85-1
- 19-Nor-4-hydroxyabieta-8,11,13-trien-7-one
Catalog No.:BCN1411
CAS No.:57906-31-7
- o-Anisic acid
Catalog No.:BCC9108
CAS No.:579-75-9
- Lobelanine
Catalog No.:BCN2156
CAS No.:579-21-5
- Oligomycin A
Catalog No.:BCC2530
CAS No.:579-13-5
- Myrianthic acid 3,23-acetonide
Catalog No.:BCN7517
CAS No.:578710-52-8
- Clozapine
Catalog No.:BCC5037
CAS No.:5786-21-0
- Idarubicin HCl
Catalog No.:BCC1194
CAS No.:57852-57-0
- Stevioside
Catalog No.:BCN6305
CAS No.:57817-89-7
- Bax inhibitor peptide P5
Catalog No.:BCC2393
CAS No.:579492-83-4
- Caffeine
Catalog No.:BCN5807
CAS No.:58-08-2
- Pyrimethamine
Catalog No.:BCC2307
CAS No.:58-14-0
- Aminophenazone
Catalog No.:BCC8815
CAS No.:58-15-1
- Methyltestosterone
Catalog No.:BCC9045
CAS No.:58-18-4
- Testosterone cypionate
Catalog No.:BCC9167
CAS No.:58-20-8
- Testosterone
Catalog No.:BCN2193
CAS No.:58-22-0
- Menadione
Catalog No.:BCN8351
CAS No.:58-27-5
- Desipramine hydrochloride
Catalog No.:BCC7553
CAS No.:58-28-6
- Promethazine HCl
Catalog No.:BCC5480
CAS No.:58-33-3
- Prochlorperazine
Catalog No.:BCC3846
CAS No.:58-38-8
- Tetrabenazine
Catalog No.:BCC5277
CAS No.:58-46-8
Regulation of cancer cell death by a novel compound, C604, in a c-Myc-overexpressing cellular environment.[Pubmed:26607468]
Eur J Pharmacol. 2015 Dec 15;769:257-65.
The proto-oncogene c-Myc has been implicated in a variety of cellular processes, such as proliferation, differentiation and apoptosis. Several c-Myc targets have been studied; however, selective regulation of c-Myc is not easy in cancer cells. Herein, we attempt to identify chemical compounds that induce cell death in c-Myc-overexpressing cells (STF-cMyc and STF-Control) by conducting MTS assays on approximately 4000 chemical compounds. One compound, C604, induced cell death in STF-cMyc cells but not STF-Control cells. Apoptotic proteins, including caspase-3 and poly(ADP-ribose) polymerase (PARP), were cleaved in C604-treated STF-cMyc cells. In addition, SW620, HCT116 and NCI-H23 cells, which exhibit higher basal levels of c-Myc, underwent apoptotic cell death in response to C604, suggesting a role for C604 as an inducer of apoptosis in cancer cells with c-Myc amplification. C604 induced cell cycle arrest at the G2/M phase in cells, which was not affected by apoptotic inhibitors. Interestingly, C604 induced accumulation of c-Myc and Cdc25A proteins. In summary, a chemical compound was identified that may induce cell death in cancer cells with c-Myc amplification specifically through an apoptotic pathway.
Cell-permeable pentapeptide V5 inhibits apoptosis and enhances insulin secretion, allowing experimental single-donor islet transplantation in mice.[Pubmed:17287463]
Diabetes. 2007 May;56(5):1259-67.
OBJECTIVE: Treatment of diabetic patients by pancreatic islet transplantation often requires the use of islets from two to four donors to produce insulin independence in a single recipient. Following isolation and transplantation, islets are susceptible to apoptosis, which limits their function and probably long-term islet graft survival. RESEARCH DESIGN AND METHODS: To address this issue, we examined the effect of the cell-permeable apoptosis inhibitor pentapeptide Val-Pro-Met-Leu-Lys, V5, on pancreatic islets in a mouse model. RESULTS: V5 treatment upregulated expression of anti-apoptotic proteins Bcl-2 and XIAP (X-linked inhibitor of apoptosis protein) by more than 3- and 11-fold and downregulated expression of apoptosis-inducing proteins Bax, Bad, and nuclear factor-kappaB-p65 by 10, 30, and nearly 50%, respectively. Treatment improved the recovered islet mass following collagenase digestion and isolation by 44% and in vitro glucose-responsive insulin secretion nearly fourfold. Following transplantation in streptozotocin-induced diabetic mice, 150 V5-treated islet equivalents functioned as well as 450 control untreated islet equivalents in normalizing blood glucose. CONCLUSIONS: These studies indicate that inhibition of apoptosis by V5 significantly improves islet function following isolation and improves islet graft function following transplantation. Use of this reagent in clinical islet transplantation could have a dramatic impact on the number of patients that might benefit from this therapy and could affect long-term graft survival.
Andrographolide sensitizes the cytotoxicity of human colorectal carcinoma cells toward cisplatin via enhancing apoptosis pathways in vitro and in vivo.[Pubmed:24563380]
Toxicol Sci. 2014 May;139(1):108-20.
Andrographolide (Andro), a diterpenoid lactone isolated from a traditional herbal medicine Andrographis paniculata, has been shown to suppress the growth and invasion of human colorectal carcinoma (CRC) Lovo cells, and trigger apoptosis in vitro. The potential of Andro as a chemotherapeutic agent in CRC was evaluated by investigating its cytotoxic effects as a single agent or in coadministration with cisplatin (CDDP). Andro potentiated the cytotoxic effect of CDDP in Lovo cells through apoptosis. The molecular mechanism for these favorable cellular response was further investigated by analyzing the apoptotic profiles, protein levels, and mRNA expression patterns of several key genes after treatments of Andro or/and CDDP. Molecular results indicated that the effect of Andro alone might be mediated via both intrinsic and extrinsic apoptotic pathways in Lovo cells. The addition of Andro to CDDP induced synergistic apoptosis, which could be corroborated to the changes in protein and mRNA levels of Bax and Bcl-2, and the increased Fas/FasL association in these cells, resulting in increased release of cytochrome c, and activation of caspases. Pretreatment of Nok-1 monoclonal antibody, a Fas signaling inhibitor, or Bax inhibitor peptide V5 repressed the Andro-induced cleavage of procaspase and the sensitization to CDDP-induced apoptosis. Finally, the combination therapy of Andro with CDDP was evidenced by its synergistic inhibition on the growth of Lovo cells in xenograft tumor studies. The results indicate that Andro, in combination with chemotherapeutics, is likely to represent a potential therapeutic strategy for CRC.
The involvement of the apoptosis-modulating proteins ERK 1/2, Bcl-xL and Bax in the resolution of acute inflammation in vivo.[Pubmed:16400007]
Am J Pathol. 2006 Jan;168(1):33-41.
Inflammatory cell recruitment, activation, and apoptosis are highly regulated processes involving several checkpoints controlling the resolution of inflammation. We investigated the role of the mitogen-activated protein kinase (MAPK) signaling pathway (ie, ERK1/2) and apoptosis-regulating Bcl-2 family members (ie, Bcl-x(L) and Bax) in the resolution of a rat carrageenan-induced pleurisy model. The specific ERK1/2 inhibitor PD98059 enhanced the resolution of inflammation, whereas the MEK1/2 inhibitor U0126 had no effect and the flavonoid apigenin, a nonspecific inhibitor of ERK1/2 and COX-2, augmented inflammation. Specifically, PD98059 significantly decreased the total number of macrophages and neutrophils in the pleural cavity, mainly by increasing the rate of neutrophil apoptosis, as measured by Annexin V labeling and morphological analysis. Conversely, a specific inhibitor of proapoptotic Bax (V5) increased inflammation, indicating that by preventing apoptosis in vivo, resolution of inflammation is delayed. This was associated with a decrease in neutrophil apoptosis and an increase in macrophage and neutrophil numbers perpetuating the inflammatory response. In conclusion, this study shows that ERK1/2, Bax, and Bcl-x(L) play important functional roles in the resolution phase of the acute inflammatory response in vivo by influencing apoptosis. Importantly, these data may provide novel therapeutic targets for the treatment of inflammatory diseases.
Overexpression of tissue factor pathway inhibitor in CHO-K1 cells results in increased activation of NF-kappaB and apoptosis mediated by a caspase-3 independent pathway.[Pubmed:22932941]
Mol Biol Rep. 2012 Dec;39(12):10089-96.
There is now circumstantial evidence that tissue factor pathway inhibitor (TFPI) is not only a major anticoagulant, but also has proapoptotic properties. The current study was designed to address the role of TFPI on signalling pathways and apoptosis. The non-TFPI expressing cell line CHO-K1 was stably transfected with pcDNA3.1/V5-His-TOPO-TFPI and control cells were established by transfecting the CHO-K1 cells with pcDNA3.1/V5-His-TOPO. Sodium butyrate (NaBut) has been shown to induce the expression of recombinant proteins. Here we have used NaBut to increase the expression of TFPI as assessed by qRT-PCR and ELISA. Compared to the control cells, TFPI induced apoptosis in a concentration dependent manner as measured by a cell death detection assay. Independent of caspase-3 activation an increased cleavage of PARP was detected in the TFPI expressing cells. This was accompanied by downregulation of Bcl-XL, elevated levels of Bax, and increased translocation of the apoptosis initiating factor. Increased DNA binding activity of NF-kappaB was revealed by electrophoretic mobility shift assay when the TFPI level was elevated by NaBut together with an increased translocation of the NF-kappaB subunit p65. The results indicate that TFPI affected the apoptotic activity through a process independent of caspase-3, and was also able to increase the activation of the NF- kappaB pathway.
Bax-inhibiting peptide derived from mouse and rat Ku70.[Pubmed:15358121]
Biochem Biophys Res Commun. 2004 Sep 3;321(4):961-6.
Bax is a proapoptotic protein that plays a key role in the induction of apoptosis. Ku70 has activities to repair DNA damage in the nucleus and to suppress apoptosis by inhibiting Bax in the cytosol. We previously designed peptides based on the amino acid sequence of Bax-binding domain of human Ku70, and showed that these peptides bind Bax and inhibit cell death in human cell lines. In the present report, we examined the biological activities of other pentapeptides, VPTLK and VPALR, derived from mouse and rat Ku70. Cells in culture accumulated FITC-labeled VPTLK and VPALR, indicating that these peptides are cell permeable (human, mouse, rat, and porcine cells were examined). These peptides bound to Bax and suppressed cell death in various cell types including primary cultured cells. These data suggest that such Bax inhibiting peptides from three mammalian species may be used to protect healthy cells from apoptotic injury under pathological conditions.


