SR 11237Pan RXR agonist CAS# 146670-40-8 |
- PA-824
Catalog No.:BCC1106
CAS No.:187235-37-6
- Clofazimine
Catalog No.:BCC4651
CAS No.:2030-63-9
- 5-hydroxypyrazine-2-carboxylic acid
Catalog No.:BCC1311
CAS No.:34604-60-9
- Nitazoxanide
Catalog No.:BCC3824
CAS No.:55981-09-4
- Sodium 4-Aminosalicylate
Catalog No.:BCC4609
CAS No.:6018-19-5
- Rifapentine
Catalog No.:BCC4937
CAS No.:61379-65-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 146670-40-8 | SDF | Download SDF |
| PubChem ID | 127019 | Appearance | Powder |
| Formula | C24H28O4 | M.Wt | 380.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BMS 649 | ||
| Solubility | Soluble to 100 mM in DMSO and to 10 mM in ethanol | ||
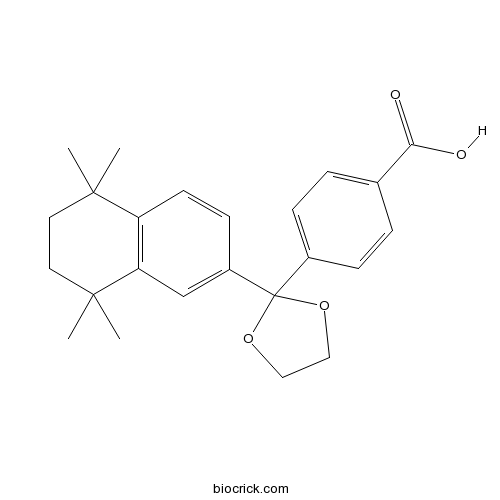
| Chemical Name | 4-[2-(5,5,8,8-tetramethyl-6,7-dihydronaphthalen-2-yl)-1,3-dioxolan-2-yl]benzoic acid | ||
| SMILES | CC1(CCC(C2=C1C=CC(=C2)C3(OCCO3)C4=CC=C(C=C4)C(=O)O)(C)C)C | ||
| Standard InChIKey | ZZUKALQMHNSWTK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H28O4/c1-22(2)11-12-23(3,4)20-15-18(9-10-19(20)22)24(27-13-14-28-24)17-7-5-16(6-8-17)21(25)26/h5-10,15H,11-14H2,1-4H3,(H,25,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pan retinoid X receptor (RXR) agonist that is devoid of any RAR activity. |

SR 11237 Dilution Calculator

SR 11237 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6283 mL | 13.1413 mL | 26.2826 mL | 52.5652 mL | 65.7065 mL |
| 5 mM | 0.5257 mL | 2.6283 mL | 5.2565 mL | 10.513 mL | 13.1413 mL |
| 10 mM | 0.2628 mL | 1.3141 mL | 2.6283 mL | 5.2565 mL | 6.5706 mL |
| 50 mM | 0.0526 mL | 0.2628 mL | 0.5257 mL | 1.0513 mL | 1.3141 mL |
| 100 mM | 0.0263 mL | 0.1314 mL | 0.2628 mL | 0.5257 mL | 0.6571 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (RS)-MCPG
Catalog No.:BCC6610
CAS No.:146669-29-6
- SR 2640 hydrochloride
Catalog No.:BCC7180
CAS No.:146662-42-2
- H-Trp(Boc)-OH
Catalog No.:BCC3115
CAS No.:146645-63-8
- Dantrolene, sodium salt
Catalog No.:BCC6673
CAS No.:14663-23-1
- 2,6-Dimethoxybenzoic acid
Catalog No.:BCN1651
CAS No.:1466-76-8
- 2-Cyclopropyl-3-[(diphenylphosphinyl)methyl]-4-(4-fluorophenyl)quinoline
Catalog No.:BCC8572
CAS No.:146578-99-6
- Fmoc-Gly(allyl)-OH
Catalog No.:BCC3156
CAS No.:146549-21-5
- Tyrphostin AG 1296
Catalog No.:BCC1195
CAS No.:146535-11-7
- WR 1065
Catalog No.:BCC2417
CAS No.:14653-77-1
- Complanatoside A
Catalog No.:BCN6282
CAS No.:146501-37-3
- 1-Methylpsilocin
Catalog No.:BCC7536
CAS No.:1465-16-3
- Pralatrexate
Catalog No.:BCC2304
CAS No.:146464-95-1
- Hybridaphniphylline A
Catalog No.:BCN7042
CAS No.:1467083-07-3
- Hybridaphniphylline B
Catalog No.:BCN7045
CAS No.:1467083-09-5
- Daphnicyclidin I
Catalog No.:BCN7038
CAS No.:1467083-10-8
- ZD 7155 hydrochloride
Catalog No.:BCC5734
CAS No.:146709-78-6
- Boc-D-Phe(4-CN)-OH
Catalog No.:BCC3183
CAS No.:146727-62-0
- N-desmethyldauricine
Catalog No.:BCC8217
CAS No.:146763-55-5
- Y-29794 oxalate
Catalog No.:BCC5795
CAS No.:146794-84-5
- 2-Bromo-1-(3-thienyl)-1-ethanone
Catalog No.:BCN2657
CAS No.:1468-82-2
- 1,5-Dihydroxyxanthone
Catalog No.:BCN7423
CAS No.:14686-65-8
- Triptobenzene H
Catalog No.:BCN6784
CAS No.:146900-55-2
- 1,2-Diacetoxy-4,7,8-trihydroxy-3-(4-hydroxyphenyl)dibenzofuran
Catalog No.:BCN7691
CAS No.:146905-24-0
- K-Ras(G12C) inhibitor 9
Catalog No.:BCC6500
CAS No.:1469337-91-4
Retinoid X receptor agonists inhibit phorbol-12-myristate-13-acetate (PMA)-induced differentiation of monocytic THP-1 cells into macrophages.[Pubmed:19784811]
Mol Cell Biochem. 2010 Feb;335(1-2):283-9.
Monocyte/macrophage differentiation is an essential process during atherosclerosis development. The retinoid X receptor (RXR) is a member of the nuclear hormone receptor superfamily, which plays an important regulatory role in many metabolic disorders, including atherosclerosis. The purpose of this study was to investigate the effect of RXR agonist on monocyte/macrophage differentiation in vitro. The THP-1 cell line was differentiated into a macrophage-like phenotype by incubation with phorbol-12-myristate-13-acetate (PMA) in the presence or absence of RXR agonist. The viability of adherent differentiated THP-1 cells was determined by MTT assay. Macrophage surface marker CD11b and CD36 was analyzed by flow cytometry. Phagocytosis was measured by fluorescence-labeled latex beads. The production of Cytokine Tunlornecrosisfactor-alpha (TNF-alpha), Interlaken-12p70 (IL-12p70), and Matrix metalloproteinase-9 (MMP-9), each of which was analyzed by ELISA. In the presence of the RXR agonists 9-cis retinoic acid or SR11237, PMA-induced THP-1 cells became less adherent, showed decreased macrophage-like morphological changes, decreased cell surface antigen CD11b and CD36 expression, and down regulated the phagocytosis of latex beads and the production of TNF-alpha and MMP-9. These data suggest that RXR agonists inhibit PMA-induced THP-1 cell differentiation into macrophage-like cells, which may be helpful in understanding the anti-atherosclerotic effect of RXR and its agonists.
RAR-independent RXR signaling induces t(15;17) leukemia cell maturation.[Pubmed:10601023]
EMBO J. 1999 Dec 15;18(24):7011-8.
Although retinoic acid receptor alpha (RARalpha) agonists induce the maturation of t(15;17) acute promyelocytic leukemia (APL) cells, drug treatment also selects leukemic blasts expressing PML-RARalpha fusion proteins with mutated ligand-binding domains that no longer respond to all-trans retinoic acid (ATRA). Here we report a novel RARalpha-independent signaling pathway that induces maturation of both ATRA-sensitive and ATRA-resistant APL NB4 cells, and does not invoke the ligand-induced alteration of PML-RARalpha signaling, stability or compartmentalization. This response involves a cross-talk between RXR agonists and protein kinase A signaling. Our results indicate the existence of a separate RXR-dependent maturation pathway that can be activated in the absence of known ligands for RXR heterodimerization partners.
Distinct retinoid X receptor-retinoic acid receptor heterodimers are differentially involved in the control of expression of retinoid target genes in F9 embryonal carcinoma cells.[Pubmed:9154799]
Mol Cell Biol. 1997 Jun;17(6):3013-20.
The F9 murine embryonal carcinoma cell line represents a well-established system for the study of retinoid signaling in vivo. We have investigated the functional specificity of different retinoid X receptor (RXR)-retinoic acid (RA) receptor (RAR) isotype pairs for the control of expression of endogenous RA-responsive genes, by using wild-type (WT), RXR alpha(-/-), RAR alpha(-/-), RAR gamma(-/-), RXR alpha(-/-)-RAR alpha(-/-), and RXR alpha(-/-)-RAR gamma(-/-) F9 cells, as well as panRXR and RAR isotype (alpha, beta, and gamma)-selective retinoids. We show that in these cells the control of expression of different sets of RA-responsive genes is preferentially mediated by distinct RXR-RAR isotype combinations. Our data support the conclusion that RXR-RAR heterodimers are the functional units transducing the retinoid signal and indicate in addition that these heterodimers exert both specific and redundant functions on the expression of particular sets of RA-responsive genes. We also show that the presence of a given receptor isotype can hinder the activity of another isotype and therefore that functional redundancy between retinoid receptor isotypes can be artifactually generated by gene knockouts.


