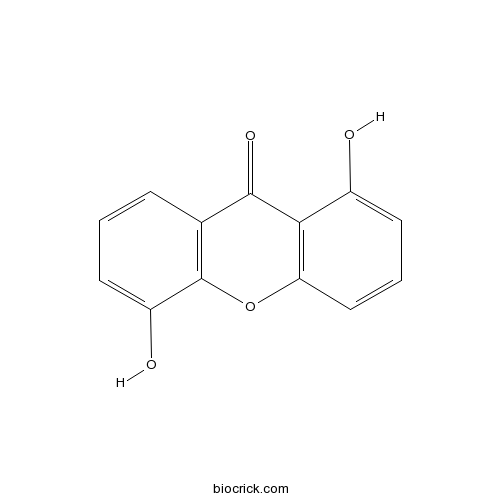
1,5-DihydroxyxanthoneCAS# 14686-65-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14686-65-8 | SDF | Download SDF |
| PubChem ID | 5480299 | Appearance | Powder |
| Formula | C13H8O4 | M.Wt | 228.20 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,5-dihydroxyxanthen-9-one | ||
| SMILES | C1=CC2=C(C(=C1)O)OC3=C(C2=O)C(=CC=C3)O | ||
| Standard InChIKey | APIPFXZYOMIJQG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H8O4/c14-8-4-2-6-10-11(8)12(16)7-3-1-5-9(15)13(7)17-10/h1-6,14-15H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 1,5-Dihydroxyxanthone may have anticholinesterase activity on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes. 2. 1,5-Dihydroxyxanthone exhibits the epidermal growth factor receptor (EGFR) -tyrosine kinase inhibitory activity, with the IC50 value of 90.34 nM. |
| Targets | AChR | BChE | EGFR |

1,5-Dihydroxyxanthone Dilution Calculator

1,5-Dihydroxyxanthone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3821 mL | 21.9106 mL | 43.8212 mL | 87.6424 mL | 109.553 mL |
| 5 mM | 0.8764 mL | 4.3821 mL | 8.7642 mL | 17.5285 mL | 21.9106 mL |
| 10 mM | 0.4382 mL | 2.1911 mL | 4.3821 mL | 8.7642 mL | 10.9553 mL |
| 50 mM | 0.0876 mL | 0.4382 mL | 0.8764 mL | 1.7528 mL | 2.1911 mL |
| 100 mM | 0.0438 mL | 0.2191 mL | 0.4382 mL | 0.8764 mL | 1.0955 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Bromo-1-(3-thienyl)-1-ethanone
Catalog No.:BCN2657
CAS No.:1468-82-2
- Y-29794 oxalate
Catalog No.:BCC5795
CAS No.:146794-84-5
- N-desmethyldauricine
Catalog No.:BCC8217
CAS No.:146763-55-5
- Boc-D-Phe(4-CN)-OH
Catalog No.:BCC3183
CAS No.:146727-62-0
- ZD 7155 hydrochloride
Catalog No.:BCC5734
CAS No.:146709-78-6
- Daphnicyclidin I
Catalog No.:BCN7038
CAS No.:1467083-10-8
- Hybridaphniphylline B
Catalog No.:BCN7045
CAS No.:1467083-09-5
- Hybridaphniphylline A
Catalog No.:BCN7042
CAS No.:1467083-07-3
- SR 11237
Catalog No.:BCC7681
CAS No.:146670-40-8
- (RS)-MCPG
Catalog No.:BCC6610
CAS No.:146669-29-6
- SR 2640 hydrochloride
Catalog No.:BCC7180
CAS No.:146662-42-2
- H-Trp(Boc)-OH
Catalog No.:BCC3115
CAS No.:146645-63-8
- Triptobenzene H
Catalog No.:BCN6784
CAS No.:146900-55-2
- 1,2-Diacetoxy-4,7,8-trihydroxy-3-(4-hydroxyphenyl)dibenzofuran
Catalog No.:BCN7691
CAS No.:146905-24-0
- K-Ras(G12C) inhibitor 9
Catalog No.:BCC6500
CAS No.:1469337-91-4
- K-Ras(G12C) inhibitor 12
Catalog No.:BCC5562
CAS No.:1469337-95-8
- Ziprasidone
Catalog No.:BCC2071
CAS No.:146939-27-7
- Codaphniphylline
Catalog No.:BCN1652
CAS No.:14694-15-6
- Fmoc-Asp(OAll)-OH
Catalog No.:BCC3089
CAS No.:146982-24-3
- Fmoc-Lys(Aloc)-OH
Catalog No.:BCC3515
CAS No.:146982-27-6
- Y-27632
Catalog No.:BCC4301
CAS No.:146986-50-7
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Atglistatin
Catalog No.:BCC5104
CAS No.:1469924-27-3
- QL47
Catalog No.:BCC3920
CAS No.:1469988-75-7
Antibacterial and EGFR-tyrosine kinase inhibitory activities of polyhydroxylated xanthones from Garcinia succifolia.[Pubmed:25460314]
Molecules. 2014 Nov 28;19(12):19923-34.
Chemical investigation of the methanol extract of the wood of Garcinia succifolia Kurz (Clusiaceae) led to the isolation of 1,5-Dihydroxyxanthone (1), 1,7-dihydroxyxanthone (2), 1,3,7-trihydroxyxanthone (3), 1,5,6-trihydroxyxanthone (4), 1,6,7-trihydroxyxanthone (5), and 1,3,6,7-tetrahydroxyxanthone (6). All of the isolated xanthones were evaluated for their antibacterial activity against bacterial reference strains, two Gram-positive (Staphylococcus aureus ATTC 25923, Bacillus subtillis ATCC 6633) and two Gram-negative (Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853), and environmental drug-resistant isolates (S. aureus B1, Enteroccoccus faecalis W1, and E. coli G1), as well as for their epidermal growth factor receptor (EGFR) of tyrosine kinase inhibitory activity. Only 1,5,6-trihydroxy-(4), 1,6,7-trihydroxy-(5), and 1,3,6,7-tetrahydroxyxanthones (6) exhibited antibacterial activity against Gram-positive bacteria, however none was active against vancomycin-resistant E. faecalis. Additionally, 1,7-dihydroxyxanthone (2) showed synergism with oxacillin, but not with ampicillin. On the other hand, only 1,5-Dihydroxyxanthone (1) and 1,7-dihydroxyxanthone (2) were found to exhibit the EGFR-tyrosine kinase inhibitory activity, with IC50 values of 90.34 and 223 nM, respectively.
Polyanxanthone A, B and C, three xanthones from the wood trunk of Garcinia polyantha Oliv.[Pubmed:18022654]
Phytochemistry. 2008 Feb;69(4):1013-7.
Three xanthones, polyanxanthone A (1), B (2) and C (3) have been isolated from the methanol extract of the wood trunk of Garcinia polyantha, along with five known xanthones: 1,3,5-trihydroxyxanthone (4); 1,5-Dihydroxyxanthone (5); 1,3,6,7-tetrahydroxyxanthone (6); 1,6-dihydroxy-5-methoxyxanthone (7) and 1,3,5,6-tetrahydroxyxanthone (8). Their structures were determined by means of 1D- and 2D-NMR techniques. Some of the above compounds were screened for their anticholinesterase activity on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes.
A new pyranoxanthone from Garcinia nervosa.[Pubmed:28412841]
Nat Prod Res. 2017 Nov;31(21):2513-2519.
Phytochemical studies on the stem bark of Garcinia nervosa has resulted in the discovery of one new pyranoxanthone derivative, garner xanthone (1) and five other compounds, 1,5-Dihydroxyxanthone (2), 6-deoxyisojacareubin (3), 12b-hydroxy-des-D-garcigerrin A (4) stigmasterol (5), and beta-sitosterol (6). The structures of these compounds were elucidated with the aid of spectroscopic techniques, such as NMR and MS. The crude extracts of the plant were assessed for their antimicrobial activity.


