Ziprasidone5-HT (serotonin)/dopamine receptor antagonist CAS# 146939-27-7 |
- Zoledronic Acid
Catalog No.:BCC1067
CAS No.:118072-93-8
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Chelerythrine chloride
Catalog No.:BCN8322
CAS No.:3895-92-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 146939-27-7 | SDF | Download SDF |
| PubChem ID | 60854 | Appearance | Powder |
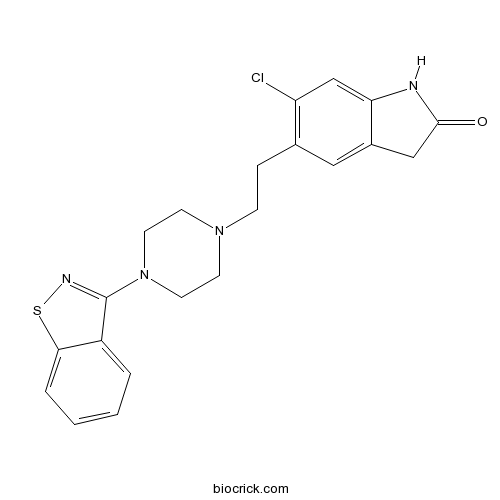
| Formula | C21H21ClN4OS | M.Wt | 412.94 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 13.5 mg/mL (32.69 mM; Need ultrasonic) | ||
| Chemical Name | 5-[2-[4-(1,2-benzothiazol-3-yl)piperazin-1-yl]ethyl]-6-chloro-1,3-dihydroindol-2-one | ||
| SMILES | C1CN(CCN1CCC2=C(C=C3C(=C2)CC(=O)N3)Cl)C4=NSC5=CC=CC=C54 | ||
| Standard InChIKey | MVWVFYHBGMAFLY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ziprasidone Dilution Calculator

Ziprasidone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4217 mL | 12.1083 mL | 24.2166 mL | 48.4332 mL | 60.5415 mL |
| 5 mM | 0.4843 mL | 2.4217 mL | 4.8433 mL | 9.6866 mL | 12.1083 mL |
| 10 mM | 0.2422 mL | 1.2108 mL | 2.4217 mL | 4.8433 mL | 6.0541 mL |
| 50 mM | 0.0484 mL | 0.2422 mL | 0.4843 mL | 0.9687 mL | 1.2108 mL |
| 100 mM | 0.0242 mL | 0.1211 mL | 0.2422 mL | 0.4843 mL | 0.6054 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ziprasidone is a combined 5-HT (serotonin) and dopamine receptor antagonist which exhibits potent effects in preclinical assays predictive of antipsychotic activity. Whereas Ziprasidone is a dopamine antagonist in vitro and in vivo, its most potent action is antagonism of 5-HT2A receptors, where its affinity is an order of magnitude greater than that observed for dopamine D2 sites. Additionally, Ziprasidone inhibits neuronal uptake of 5-HT and norepinephrine comparable to the antidepressant imipramine. Ziprasidone differs from other atypical antipsychotic drugs in several clinically important ways, although further experience is necessary to clarify the significance of these differences.
- K-Ras(G12C) inhibitor 12
Catalog No.:BCC5562
CAS No.:1469337-95-8
- K-Ras(G12C) inhibitor 9
Catalog No.:BCC6500
CAS No.:1469337-91-4
- 1,2-Diacetoxy-4,7,8-trihydroxy-3-(4-hydroxyphenyl)dibenzofuran
Catalog No.:BCN7691
CAS No.:146905-24-0
- Triptobenzene H
Catalog No.:BCN6784
CAS No.:146900-55-2
- 1,5-Dihydroxyxanthone
Catalog No.:BCN7423
CAS No.:14686-65-8
- 2-Bromo-1-(3-thienyl)-1-ethanone
Catalog No.:BCN2657
CAS No.:1468-82-2
- Y-29794 oxalate
Catalog No.:BCC5795
CAS No.:146794-84-5
- N-desmethyldauricine
Catalog No.:BCC8217
CAS No.:146763-55-5
- Boc-D-Phe(4-CN)-OH
Catalog No.:BCC3183
CAS No.:146727-62-0
- ZD 7155 hydrochloride
Catalog No.:BCC5734
CAS No.:146709-78-6
- Daphnicyclidin I
Catalog No.:BCN7038
CAS No.:1467083-10-8
- Hybridaphniphylline B
Catalog No.:BCN7045
CAS No.:1467083-09-5
- Codaphniphylline
Catalog No.:BCN1652
CAS No.:14694-15-6
- Fmoc-Asp(OAll)-OH
Catalog No.:BCC3089
CAS No.:146982-24-3
- Fmoc-Lys(Aloc)-OH
Catalog No.:BCC3515
CAS No.:146982-27-6
- Y-27632
Catalog No.:BCC4301
CAS No.:146986-50-7
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Atglistatin
Catalog No.:BCC5104
CAS No.:1469924-27-3
- QL47
Catalog No.:BCC3920
CAS No.:1469988-75-7
- Diphenhydramine hydrochloride
Catalog No.:BCC8947
CAS No.:147-24-0
- DL-Arabinose
Catalog No.:BCN8541
CAS No.:147-81-9
- Proline
Catalog No.:BCN1656
CAS No.:147-85-3
- Cytarabine
Catalog No.:BCC3759
CAS No.:147-94-4
- 3'-O-Demethylarctigenin
Catalog No.:BCN3544
CAS No.:147022-95-5
Intramuscular Ziprasidone Dosing for Acute Agitation in the Pediatric Emergency Department: An Observational Study.[Pubmed:28205446]
J Pharm Pract. 2018 Feb;31(1):18-21.
BACKGROUND: Intramuscular (IM) Ziprasidone is often used to manage acute agitation. Limited data exist on the pediatric dosing of Ziprasidone in the emergency department (ED). OBJECTIVE: To characterize the mg/kg dosing differences between pediatric ED patients who respond to an initial dose of Ziprasidone versus patients who do not. METHODS: This was a retrospective, observational study of 5- to 18-year-old patients who were treated with IM Ziprasidone in the pediatric ED from 2007 to 2015. Medical records were reviewed to determine demographic and clinical information. Patients were deemed responders to Ziprasidone if they required no additional rescue medication for acute agitation within 30 minutes of the initial dose. RESULTS: Forty children received 50 doses of IM Ziprasidone. Twenty-seven (68%) patients responded to the initial Ziprasidone dose, requiring no further medication intervention for their acute agitation. Responders were given a mean initial dose of 0.19 +/- 0.1 mg/kg, while nonresponders were given an initial mean dose of 0.13 +/- 0.06 mg/kg ( P = .03). CONCLUSION: A significant dose difference exists between patients who required only one initial dose of Ziprasidone compared to those who required additional medication. As a result, an initial dose of 0.2 mg/kg of IM Ziprasidone may be considered when managing acutely agitated pediatric patients in the ED.
Effects of aripiprazole, quetiapine and ziprasidone on plasma prolactin levels in individuals with first episode nonaffective psychosis: Analysis of a randomized open-label 1year study.[Pubmed:28223031]
Schizophr Res. 2017 Nov;189:134-141.
RATIONALE: Hyperprolactinemia is considered a troubling adverse effect of antipsychotics. Direct comparisons among second generation antipsychotics are scant in clinical practice. We hypothesize prolactin-sparing second-generation antipsychotics may have differential effects on prolactin levels and that they may be influenced by sex. OBJECTIVES: To explore the differential effect of three widely used prolactin-sparing antipsychotics, aripiprazole, quetiapine and Ziprasidone, on prolactin plasma levels in first episode non-affective psychosis during a 1year of treatment. METHOD: From October 2005 to January 2011 a prospective, randomized, open-label study was undertaken. 141 patients who were randomly allocated to aripiprazole (N=56), quetiapine (N=36) or Ziprasidone (N=49) were analyzed. The main outcome was differences in prolactin plasma levels over 1year follow-up among the three antipsychotics. Prolactin levels had a skewed distribution and therefore they were log-transformed before statistical analyses. RESULTS: Male patients on aripiprazole had a lower risk of suffering an increase on prolactin plasma levels (N=71; F=12.645; p<0.001). There was a gender effect with smaller changes in mean prolactin values only in males. Aripiprazole had a reduced risk of hyperprolactinemia (aripiprazole 19.6%) compared to quetiapine (44.4%) and Ziprasidone (32.7%) (p=0.038); and quite similar findings were found when investigating males (p=0.040). No significant differences were found in females. The percentages of mild prolactin excess were: 14.3% on aripiprazole, 36.1% on quetiapine and 18.4% on Ziprasidone (chi(2)=6.611 p=0.037). CONCLUSIONS: Our findings provide additional evidence of differential effects of three sparing-prolactin antipsychotics on prolactin release and may help clinicians to decide among therapeutic options.
The impact of gastric pH, volume, and emptying on the food effect of ziprasidone oral absorption.[Pubmed:28321831]
AAPS J. 2017 Jul;19(4):1084-1090.
In a recent food effect clinical study, the authors concluded that a meal consisting of >/=500 kcal, regardless of fat content, produced the maximal bioavailability for Ziprasidone. Using GastroPlus, a commercially available pharmacokinetic simulation software, a semiphysiological model-a kind of physiologically based pharmacokinetic (PBPK) absorption model-was developed that could predict the concentration-time profiles when Ziprasidone was administered with any one of the five test meals or fasting. Ziprasidone intravenous pharmacokinetics and oral absorption permeability were determined from clinical studies following the intravenous and duodenal infusion of Ziprasidone to volunteers. From the detailed dietary information of each meal provided in the previously published food effect study, the stomach pH, volume, and gastric emptying could be predicted. Incorporating these meal-specific parameters into the model improved the predictions beyond the default fed/fasted parameters commonly used in the software. Compared to the default models, the improved models resulted in an improved prediction of the average Ziprasidone concentration-time profile for each meal. Using this type of semiphysiological absorption model, we have shown that the dietary contents of the meals should be taken into account to predict food effects for Ziprasidone and perhaps other BCS class I or II compounds.
A neuroimaging study of emotion-cognition interaction in schizophrenia: the effect of ziprasidone treatment.[Pubmed:28210783]
Psychopharmacology (Berl). 2017 Apr;234(7):1045-1058.
Functional and structural brain changes associated with the cognitive processing of emotional visual stimuli were assessed in schizophrenic patients after 16 weeks of antipsychotic treatment with Ziprasidone. Forty-five adults aged 18 to 40 were recruited: 15 schizophrenia patients (DSM-IV criteria) treated with Ziprasidone (mean daily dose = 120 mg), 15 patients treated with other antipsychotics, and 15 healthy controls who did not receive any medication. Functional and structural neuroimaging data were acquired at baseline and 16 weeks after treatment initiation. In each session, participants selected stimuli, taken from standardized sets, based on their emotional valence. After Ziprasidone treatment, several prefrontal regions, typically involved in cognitive control (anterior cingulate and ventrolateral prefrontal cortices), were significantly activated in patients in response to positive versus negative stimuli. This effect was greater whenever they had to select negative compared to positive stimuli, indicating an asymmetric effect of cognitive treatment of emotionally laden information. No such changes were observed for patients under other antipsychotics. In addition, there was an increase in the brain volume commonly recruited by healthy controls and patients under Ziprasidone, in response to cognitive processing of emotional information. The structural analysis showed no significant changes in the density of gray and white matter in Ziprasidone-treated patients compared to patients receiving other antipsychotic treatments. Our results suggest that functional changes in brain activity after Ziprasidone medication precede structural and clinical manifestations, as markers that the treatment is efficient in restoring the functionality of prefrontal circuits involved in processing emotionally laden information in schizophrenia.


