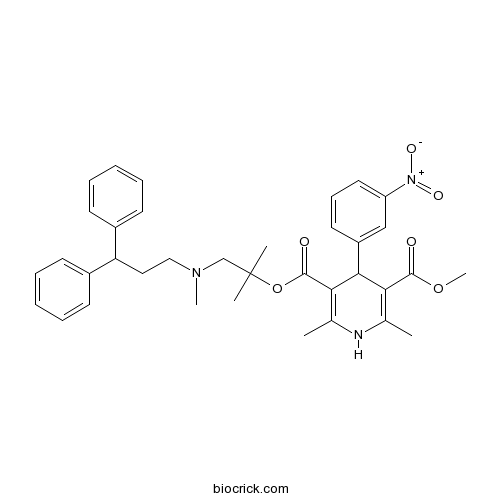
LercanidipineCAS# 100427-26-7 |
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- Necrostatin 2 racemate
Catalog No.:BCC2077
CAS No.:852391-15-2
- Necrostatin 2
Catalog No.:BCC1793
CAS No.:852391-19-6
- Necrostatin 2 S enantiomer
Catalog No.:BCC2078
CAS No.:852391-20-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 100427-26-7 | SDF | Download SDF |
| PubChem ID | 65866 | Appearance | Powder |
| Formula | C36H41N3O6 | M.Wt | 611.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | 5-O-[1-[3,3-diphenylpropyl(methyl)amino]-2-methylpropan-2-yl] 3-O-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate | ||
| SMILES | CC1=C(C(C(=C(N1)C)C(=O)OC(C)(C)CN(C)CCC(C2=CC=CC=C2)C3=CC=CC=C3)C4=CC(=CC=C4)[N+](=O)[O-])C(=O)OC | ||
| Standard InChIKey | ZDXUKAKRHYTAKV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C36H41N3O6/c1-24-31(34(40)44-6)33(28-18-13-19-29(22-28)39(42)43)32(25(2)37-24)35(41)45-36(3,4)23-38(5)21-20-30(26-14-9-7-10-15-26)27-16-11-8-12-17-27/h7-19,22,30,33,37H,20-21,23H2,1-6H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Lercanidipine Dilution Calculator

Lercanidipine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6347 mL | 8.1735 mL | 16.3471 mL | 32.6942 mL | 40.8677 mL |
| 5 mM | 0.3269 mL | 1.6347 mL | 3.2694 mL | 6.5388 mL | 8.1735 mL |
| 10 mM | 0.1635 mL | 0.8174 mL | 1.6347 mL | 3.2694 mL | 4.0868 mL |
| 50 mM | 0.0327 mL | 0.1635 mL | 0.3269 mL | 0.6539 mL | 0.8174 mL |
| 100 mM | 0.0163 mL | 0.0817 mL | 0.1635 mL | 0.3269 mL | 0.4087 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Danshinspiroketallactone
Catalog No.:BCN3754
CAS No.:100414-80-0
- Sodium Picosulfate
Catalog No.:BCC4845
CAS No.:10040-45-6
- GSK 1562590 hydrochloride
Catalog No.:BCC8010
CAS No.:1003878-07-6
- Calcium chloride dihydrate
Catalog No.:BCC7582
CAS No.:10035-04-8
- Curcumenone
Catalog No.:BCN3008
CAS No.:100347-96-4
- L(+)-Rhamnose monohydrate
Catalog No.:BCN8368
CAS No.:10030-85-0
- Pemirolast potassium
Catalog No.:BCC4532
CAS No.:100299-08-9
- Apiopaeonoside
Catalog No.:BCN2801
CAS No.:100291-86-9
- Irinotecan hydrochloride
Catalog No.:BCN2949
CAS No.:100286-90-6
- TZ9
Catalog No.:BCC5547
CAS No.:1002789-86-7
- Picrasidine J
Catalog No.:BCN5820
CAS No.:100234-62-6
- Picrasidine I
Catalog No.:BCN5819
CAS No.:100234-59-1
- Boric acid
Catalog No.:BCC7592
CAS No.:10043-35-3
- Cobicistat (GS-9350)
Catalog No.:BCC2271
CAS No.:1004316-88-4
- Dihydroresveratrol 3-O-glucoside
Catalog No.:BCN5821
CAS No.:100432-87-9
- 1-EBIO
Catalog No.:BCC6904
CAS No.:10045-45-1
- FFN 511
Catalog No.:BCC7799
CAS No.:1004548-96-2
- Rosiridin
Catalog No.:BCN5970
CAS No.:100462-37-1
- Gastrin I (human)
Catalog No.:BCC5958
CAS No.:10047-33-3
- Sterigmatocystin
Catalog No.:BCN6885
CAS No.:10048-13-2
- Blasticidin A
Catalog No.:BCN1830
CAS No.:100513-53-9
- TCS 2002
Catalog No.:BCC6074
CAS No.:1005201-24-0
- Aeruginolactone
Catalog No.:BCN3695
CAS No.:1005208-88-7
- Gelomulide N
Catalog No.:BCN6641
CAS No.:1005212-02-1
Replacement of Amlodipine and Lercanidipine by Barnidipine: Tolerability and Effectiveness in a Real-Life Study.[Pubmed:28058623]
High Blood Press Cardiovasc Prev. 2017 Mar;24(1):29-36.
INTRODUCTION: Hypertension is the leading cause of cardiovascular disease worldwide. Calcium channel blockers are an effective antihypertensive treatment, but frequently hypertension remains uncontrolled for many patients, partly due to tolerability issues. AIM: To assess the tolerability and effectiveness of barnidipine in mild to moderate hypertension patients switching treatment from other calcium channel blockers in daily practice. METHODS: BASIC-HT, a prospective real life study, enrolled 20,479 hypertensive patients initiating barnidipine treatment. The present paper focuses on a subgroup of patients in BASIC-HT for whom the previous treatment with amlodipine or Lercanidipine was replaced by barnidipine. Tolerability and effectiveness of barnidipine in these patients were assessed at two visits during a 3-month follow up. RESULTS: In 1710 mild to moderate hypertension patients switching treatment from amlodipine or Lercanidipine to barnidipine monotherapy or in combination with other antihypertensive drug classes, mean blood pressure decreased during 3-month follow-up. The mean systolic blood pressure decreased from 153.15 mmHg [95% CI 152.35-153.95] at baseline to 139.20 mmHg [95% CI 138.58-139.82] at visit 3, after 3 months. The mean diastolic blood pressure decreased from 88.85 mmHg at baseline [95% CI 88.36-89.34], to 81.56 mmHg [95% CI 81.20-81.91] at visit 3. Among these patients, 65.4% replaced their initial calcium channel blocker treatment to barnidipine for tolerability reasons. During the follow-up, the main adverse event reported was edema (4.8%). The nature and frequency of events reported in this subgroup of switcher patients were in line with those reported by the total population in BASIC-HT. CONCLUSION: This real-life study suggests that replacement of other calcium channel blockers with barnidipine is a valuable therapeutic option, especially when tolerability is an issue.
Combination therapy with lercanidipine and enalapril in the management of the hypertensive patient: an update of the evidence.[Pubmed:27895487]
Vasc Health Risk Manag. 2016 Nov 15;12:443-451.
Hypertension is an important risk factor for premature death as it increases the probability of stroke, myocardial infarction, and heart failure. Antihypertensive drugs can decrease cardiovascular (CV) morbidity and mortality. The majority of hypertensive patients need more than one antihypertensive agent to attain blood pressure (BP) targets. Monotherapy can effectively reduce BP only in 20%-40% of patients. Multiple mechanisms including increased peripheral vascular resistance, increased cardiac work, and hypervolemia are involved in the pathogenesis of hypertension. Targeting multiple pathways may more potently reduce BP. Increasing the dose of a single agent in many cases does not provide the expected BP-lowering effect because the underlying mechanism of the BP increase is either different or already corrected with the lower dose. Moreover, drugs acting on different pathways may have synergistic effects and thus better control hypertension. It is well known that diuretics enhance the actions of renin-angiotensin aldosterone system and activate it as a feedback to the reduced circulated blood volume. The addition of a renin-angiotensin aldosterone system blocker to a diuretic may more effectively reduce BP because the system is upregulated. Reducing the maximal dose of an agent may also reduce possible side effects if they are dose dependent. The increased prevalence of peripheral edema with higher doses of calcium channel blockers (CCBs) is reduced when renin-angiotensin aldosterone system blockers are added to CCBs through vein dilation. The effectiveness of the combination of enalapril with Lercanidipine in reducing BP, the safety profile, and the use of the combination of angiotensin-converting enzyme inhibitors with CCBs in clinical trials with excellent CV hard end point outcomes make this combination a promising therapy in the treatment of hypertension.
Neuroprotective effect of lercanidipine in middle cerebral artery occlusion model of stroke in rats.[Pubmed:27794423]
Exp Neurol. 2017 Feb;288:25-37.
Oxidative stress, inflammation and apoptotic neuronal cell death are cardinal mechanisms involved in the cascade of acute ischemic stroke. Lercanidipine apart from calcium channel blocking activity possesses anti-oxidant, anti-inflammatory and anti-apoptotic properties. In the present study, we investigated neuroprotective efficacy and therapeutic time window of Lercanidipine in a 2h middle cerebral artery occlusion (MCAo) model in male Wistar rats. The study design included: acute (pre-treatment and post-treatment) and sub-acute studies. In acute studies (pre-treatment) Lercanidipine (0.25, 0.5 and 1mg/kg, i.p.) was administered 60min prior MCAo. The rats were assessed 24h post-MCAo for neurological deficit score (NDS), motor deficit paradigms (grip test and rota rod) and cerebral infarction via 2,3,5-triphenyltetrazolium chloride (TTC) staining. The most effective dose was found to be at 0.5mg/kg, i.p., which was considered for further studies. Regional cerebral blood flow (rCBF) was monitored till 120min post-reperfusion to assess vasodilatory property of Lercanidipine (0.5mg/kg, i.p.) administered at two different time points: 60min post-MCAo and 15min post-reperfusion. In acute studies (post-treatment) Lercanidipine (0.5mg/kg, i.p.) was administered 15min, 120min and 240min post-reperfusion. Based on NDS and cerebral infarction via TTC staining assessed 24h post-MCAo, effectiveness was evident upto 120min. For sub-acute studies same dose/vehicle was repeated for next 3days and magnetic resonance imaging (MRI) was performed 96h after the last dose. Biochemical markers estimated in rat brain cortex 24h post-MCAo were oxidative stress (malondialdehyde, reduced glutathione, nitric oxide, superoxide dismutase), blood brain barrier damage (matrix metalloproteinases-2 and -9) and apoptotic (caspase-3 and -9). Lercanidipine significantly reduced NDS, motor deficits and cerebral infarction volume as compared to the control group. Lercanidipine (60min post-MCAo) significantly increased rCBF (86%) as compared to vehicle treated MCAo group (64%) 120min post-reperfusion, but failed to show vasodilatation with 15min post-reperfusion group. Lercanidipine (13.78+/-2.78%) significantly attenuated percentage infarct volume as evident from diffusion-weighted (DWI) and T2-weighted images as compared to vehicle treated MCAo group (25.90+/-2.44%) investigated 96h post-MCAo. The apparent diffusion coefficient (ADC) was also significantly improved in Lercanidipine group as compared to control group. Biochemical alterations were significantly ameliorated by Lercanidipine till 120min post-reperfusion group and MMP-9 inhibition observed even with 240min group. Thus, Lercanidipine revealed significant neuroprotective effect mediated through attenuation of oxidative stress, inflammation and apoptosis.
Long-term outcomes of lercanidipine versus other calcium channel blockers in newly diagnosed hypertension: a nationwide cohort study.[Pubmed:28300435]
Curr Med Res Opin. 2017 Jun;33(6):1111-1117.
OBJECTIVE: Calcium channel blockers (CCBs) have been proved to have beneficial effects on cardiovascular (CV) outcomes, especially in stroke. Lercanidipine, a highly lipophilic CCB, lacks data regarding long-term outcomes including: CV, stroke, renal and all-cause mortality. This retrospective cohort study aims to clarify this. PATIENTS AND METHODS: A total of 144,630 newly diagnosed hypertension (HTN) patients (age: 18-65 years) in 2005 from the Taiwan's National Health Insurance Research Database were enrolled in this observational study. A pure hypertension population was fetched by excluding all chronic diseases in the Charlson Comorbidities Index. Patients were stratified into the Lercanidipine group (n = 1303) and the propensity-score-matched comparative group (nifedipine, amlodipine or felodipine, n = 15,301). RESULTS: Compared to other CCBs, Lercanidipine didn't have a significant difference on the study endpoints. In individual head-to-head comparisons, Lercanidipine was shown to be superior to nifedipine in incident stroke with an adjusted HR with 95% CI of 0.526 (0.347-0.797) (p = .0025). The key limitations were that personal variables, such as smoking habits, alcohol intake, body mass index and physical activity and blood pressure profiles were not available in the nationwide registry database. CONCLUSION: In newly diagnosed patients with hypertension, Lercanidipine was superior to nifedipine in the six-year period when the analyzed endpoint was stroke.


