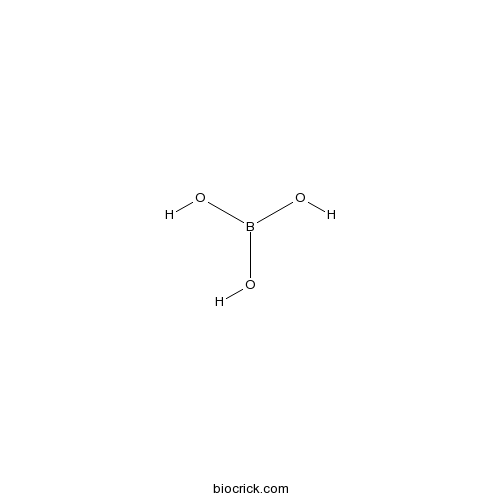
Boric acidCAS# 10043-35-3 |
- MK-5172 hydrate
Catalog No.:BCC1763
CAS No.:1350462-55-3
- Telaprevir (VX-950)
Catalog No.:BCC2107
CAS No.:402957-28-2
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Danoprevir (RG7227)
Catalog No.:BCC2106
CAS No.:850876-88-9
- Narlaprevir
Catalog No.:BCC1785
CAS No.:865466-24-6
- Simeprevir
Catalog No.:BCC1949
CAS No.:923604-59-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10043-35-3 | SDF | Download SDF |
| PubChem ID | 7628 | Appearance | Powder |
| Formula | H3BO3 | M.Wt | 61.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 600 mM in water | ||
| Chemical Name | boric acid | ||
| SMILES | B(O)(O)O | ||
| Standard InChIKey | KGBXLFKZBHKPEV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/BH3O3/c2-1(3)4/h2-4H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Widely used in buffers for electrophoresis. |

Boric acid Dilution Calculator

Boric acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 16.1734 mL | 80.8669 mL | 161.7338 mL | 323.4676 mL | 404.3345 mL |
| 5 mM | 3.2347 mL | 16.1734 mL | 32.3468 mL | 64.6935 mL | 80.8669 mL |
| 10 mM | 1.6173 mL | 8.0867 mL | 16.1734 mL | 32.3468 mL | 40.4334 mL |
| 50 mM | 0.3235 mL | 1.6173 mL | 3.2347 mL | 6.4694 mL | 8.0867 mL |
| 100 mM | 0.1617 mL | 0.8087 mL | 1.6173 mL | 3.2347 mL | 4.0433 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lercanidipine
Catalog No.:BCC5239
CAS No.:100427-26-7
- Danshinspiroketallactone
Catalog No.:BCN3754
CAS No.:100414-80-0
- Sodium Picosulfate
Catalog No.:BCC4845
CAS No.:10040-45-6
- GSK 1562590 hydrochloride
Catalog No.:BCC8010
CAS No.:1003878-07-6
- Calcium chloride dihydrate
Catalog No.:BCC7582
CAS No.:10035-04-8
- Curcumenone
Catalog No.:BCN3008
CAS No.:100347-96-4
- L(+)-Rhamnose monohydrate
Catalog No.:BCN8368
CAS No.:10030-85-0
- Pemirolast potassium
Catalog No.:BCC4532
CAS No.:100299-08-9
- Apiopaeonoside
Catalog No.:BCN2801
CAS No.:100291-86-9
- Irinotecan hydrochloride
Catalog No.:BCN2949
CAS No.:100286-90-6
- TZ9
Catalog No.:BCC5547
CAS No.:1002789-86-7
- Picrasidine J
Catalog No.:BCN5820
CAS No.:100234-62-6
- Cobicistat (GS-9350)
Catalog No.:BCC2271
CAS No.:1004316-88-4
- Dihydroresveratrol 3-O-glucoside
Catalog No.:BCN5821
CAS No.:100432-87-9
- 1-EBIO
Catalog No.:BCC6904
CAS No.:10045-45-1
- FFN 511
Catalog No.:BCC7799
CAS No.:1004548-96-2
- Rosiridin
Catalog No.:BCN5970
CAS No.:100462-37-1
- Gastrin I (human)
Catalog No.:BCC5958
CAS No.:10047-33-3
- Sterigmatocystin
Catalog No.:BCN6885
CAS No.:10048-13-2
- Blasticidin A
Catalog No.:BCN1830
CAS No.:100513-53-9
- TCS 2002
Catalog No.:BCC6074
CAS No.:1005201-24-0
- Aeruginolactone
Catalog No.:BCN3695
CAS No.:1005208-88-7
- Gelomulide N
Catalog No.:BCN6641
CAS No.:1005212-02-1
- CVT 10216
Catalog No.:BCC5606
CAS No.:1005334-57-5
Polar Localization of the NIP5;1 Boric Acid Channel Is Maintained by Endocytosis and Facilitates Boron Transport in Arabidopsis Roots.[Pubmed:28341806]
Plant Cell. 2017 Apr;29(4):824-842.
Boron uptake in Arabidopsis thaliana is mediated by nodulin 26-like intrinsic protein 5;1 (NIP5;1), a Boric acid channel that is located preferentially on the soil side of the plasma membrane in root cells. However, the mechanism underlying this polar localization is poorly understood. Here, we show that the polar localization of NIP5;1 in epidermal and endodermal root cells is mediated by the phosphorylation of Thr residues in the conserved TPG (ThrProGly) repeat in the N-terminal region of NIP5;1. Although substitutions of Ala for three Thr residues in the TPG repeat did not affect lateral diffusion in the plasma membrane, these substitutions inhibited endocytosis and strongly compromised the polar localization of GFP-NIP5;1. Consistent with this, the polar localization was compromised in micro subunit mutants of the clathrin adaptor AP2. The Thr-to-Ala substitutions did not affect the boron transport activity of GFP-NIP5;1 in Xenopus laevis oocytes but did inhibit the ability to complement boron translocation to shoots and rescue growth defects in nip5;1-1 mutant plants under boron-limited conditions. These results demonstrate that the polar localization of NIP5;1 is maintained by clathrin-mediated endocytosis, is dependent on phosphorylation in the TPG repeat, and is necessary for the efficient transport of boron in roots.
Boric acid-destabilized lithium borohydride with a 5.6 wt% dehydrogenation capacity at moderate temperatures.[Pubmed:28300266]
Dalton Trans. 2017 Apr 5;46(14):4499-4503.
Boric acid effectively promotes the dehydrogenation of lithium borohydride due to the interactions between protonic and hydridic hydrogen. The simple mixture of LiBH4-(4/3)B(OH)3 can release 5.6 wt% hydrogen below 180 degrees C with only a trace amount of water, thus constituting a highly attractive single-use hydrogen storage material.
Ecotoxicity of boric acid in standard laboratory tests with plants and soil organisms.[Pubmed:28314961]
Ecotoxicology. 2017 May;26(4):471-481.
To verify the continuous sensitivity of ecotoxicological tests (mainly the test organisms), reference substances with known toxicity are regularly tested. Ideally, this substance(s) would lack specificity in its mode action, be bioavailable and readily attainable with cost-effective means of chemical characterization. Boric acid has satisfied these criteria, but has most recently been characterized as a substance of very high concern, due to reproductive effects in humans, thus limiting its recommendation as an ideal reference toxicant. However, there is probably no other chemical for which ecotoxicity in soil has been so intensively studied; an extensive literature review yielded lethal (including avoidance) and sublethal data for 38 taxa. The ecotoxicity data were evaluated using species sensitivity distributions, collectively across all taxa, and separately according to species type, endpoints, soil type and duration. The lack of specificity in the mode of action yielded broad toxicity among soil taxa and soil types, and provided a collective approach to assessing species sensitivity, while taking into consideration differences in test methodologies and exposure durations. Toxicity was species-specific with Folsomia candida and enchytraied species demonstrating the most sensitivity; among plants, the following trend occurred: dicotyledonous (more sensitive) >> monocotyledonous >> gymnosperm species. Sensitivity was also time and endpoint specific, with endpoints such as lethality and avoidance being less sensitive than reproduction effects. Furthermore, given the breadth of data and toxicity demonstrated by Boric acid, lessons learned from its evaluation are discussed to recommend the properties required by an ideal reference substance for the soil compartment.


