TZ9CAS# 1002789-86-7 |
- DCC-2618
Catalog No.:BCC1520
CAS No.:1225278-16-9
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Sorafenib
Catalog No.:BCN2174
CAS No.:284461-73-0
- Pazopanib (GW-786034)
Catalog No.:BCC1286
CAS No.:444731-52-6
- Masitinib (AB1010)
Catalog No.:BCC1260
CAS No.:790299-79-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1002789-86-7 | SDF | Download SDF |
| PubChem ID | 7593228 | Appearance | Powder |
| Formula | C17H14N6O4 | M.Wt | 366.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 40 mg/mL (109.19 mM) *"≥" means soluble, but saturation unknown. | ||
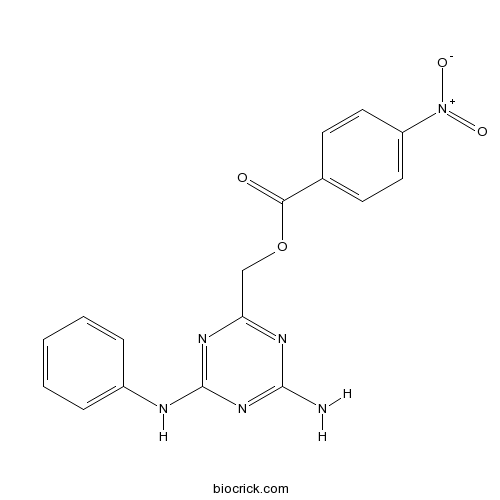
| Chemical Name | (4-amino-6-anilino-1,3,5-triazin-2-yl)methyl 4-nitrobenzoate | ||
| SMILES | C1=CC=C(C=C1)NC2=NC(=NC(=N2)N)COC(=O)C3=CC=C(C=C3)[N+](=O)[O-] | ||
| Standard InChIKey | RRRDZFQRNJTKHL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14N6O4/c18-16-20-14(21-17(22-16)19-12-4-2-1-3-5-12)10-27-15(24)11-6-8-13(9-7-11)23(25)26/h1-9H,10H2,(H3,18,19,20,21,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

TZ9 Dilution Calculator

TZ9 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7298 mL | 13.6489 mL | 27.2978 mL | 54.5956 mL | 68.2445 mL |
| 5 mM | 0.546 mL | 2.7298 mL | 5.4596 mL | 10.9191 mL | 13.6489 mL |
| 10 mM | 0.273 mL | 1.3649 mL | 2.7298 mL | 5.4596 mL | 6.8244 mL |
| 50 mM | 0.0546 mL | 0.273 mL | 0.546 mL | 1.0919 mL | 1.3649 mL |
| 100 mM | 0.0273 mL | 0.1365 mL | 0.273 mL | 0.546 mL | 0.6824 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
TZ9 is a novel inhibitor of Rad6 ubiquitin conjugating enzyme(E2 enzyme); inhibits MDA-MB-231 cell proliferation with IC50 of ~6 uM. IC50 value: 6 uM [1] Target: Rad6 inhibitor The bulk of MDA-MB-231 cells treated with 10 μmol/L or more SMI #9 displayed a round morphology compared with controls and less than 5 μmol/L doses of SMI #9. Simultaneous comparison of SMIs #8 and 9 confirmed SMI #9 inhibits Matrigel-induced migration of MDA-MB-231 cells in a dose-dependent manner compared with SMI #8. 5 μmol/L SMI #9 treatment triggered morphologic changes consistent with apoptosis in a time-dependent manner. 5 μmol/L SMI #9 treatment of MDA-MB-231 cells for 24 hours increased the proportion of G2–M-arrested cells by 2-fold and was accompanied by a proportional decrease in S-phase cells. SMIs #8 or 9 treatments dramatically reduced β-catenin staining as visualized by reduced merged Rad6/β-catenin yellow fluorescence.
References:
[1]. Sanders MA, et al. Novel inhibitors of Rad6 ubiquitin conjugating enzyme: design, synthesis, identification, and functional characterization. Mol Cancer Ther. 2013 Apr;12(4):373-83.
- Picrasidine J
Catalog No.:BCN5820
CAS No.:100234-62-6
- Picrasidine I
Catalog No.:BCN5819
CAS No.:100234-59-1
- AMG-208
Catalog No.:BCC1054
CAS No.:1002304-34-8
- Camstatin
Catalog No.:BCC5690
CAS No.:1002295-95-5
- 5,5'-Dimethoxysecoisolariciresinol
Catalog No.:BCN7941
CAS No.:1002106-91-3
- 2,3-dihydroxy-3-(4-hydroxyphenyl)propanoic acid
Catalog No.:BCN1641
CAS No.:100201-57-8
- Piscidinol A
Catalog No.:BCN5818
CAS No.:100198-09-2
- MK-8033
Catalog No.:BCC1768
CAS No.:1001917-37-8
- INH6
Catalog No.:BCC5455
CAS No.:1001753-24-7
- SRT1720 HCl
Catalog No.:BCC2222
CAS No.:1001645-58-4
- BV6
Catalog No.:BCC5372
CAS No.:1001600-56-1
- 1,4,5,6-Tetrahydroxy-7-prenylxanthone
Catalog No.:BCN1642
CAS No.:1001424-68-5
- Irinotecan hydrochloride
Catalog No.:BCN2949
CAS No.:100286-90-6
- Apiopaeonoside
Catalog No.:BCN2801
CAS No.:100291-86-9
- Pemirolast potassium
Catalog No.:BCC4532
CAS No.:100299-08-9
- L(+)-Rhamnose monohydrate
Catalog No.:BCN8368
CAS No.:10030-85-0
- Curcumenone
Catalog No.:BCN3008
CAS No.:100347-96-4
- Calcium chloride dihydrate
Catalog No.:BCC7582
CAS No.:10035-04-8
- GSK 1562590 hydrochloride
Catalog No.:BCC8010
CAS No.:1003878-07-6
- Sodium Picosulfate
Catalog No.:BCC4845
CAS No.:10040-45-6
- Danshinspiroketallactone
Catalog No.:BCN3754
CAS No.:100414-80-0
- Lercanidipine
Catalog No.:BCC5239
CAS No.:100427-26-7
- Boric acid
Catalog No.:BCC7592
CAS No.:10043-35-3
- Cobicistat (GS-9350)
Catalog No.:BCC2271
CAS No.:1004316-88-4
Design, synthesis and characterization of doped-titanium oxide nanomaterials with environmental and angiogenic applications.[Pubmed:28525935]
Sci Total Environ. 2017 Dec 1;599-600:1263-1274.
Since the last decade, the metal composite nanostructures have evolved as promising candidates in regard to their wide applications in the fields of science and engineering. Recently, several investigators identified the titanium based nanomaterials as excellent agents for multifunctional environmental and biomedical applications. In this perspective, we have developed a series of zinc-doped (2 and 5%) titanium oxide-based nanomaterials using various reaction conditions and calcination temperatures (TZ1-TZ3: calcined at 500 degrees C, TZ4-TZ6: calcined at 600 degrees C and TZ7-TZ9: calcined at 700 degrees C). The calcined materials (TZ1 to TZ9) were thoroughly analyzed by several physico-chemical characterization methods. The increase of the calcination temperature results in significant changes of the textural properties of the nanostructured materials. In addition, the increase of the calcination temperature leads to the formation of anatase/rutile mixtures with higher quantity of rutile. Furthermore, incorporation of zinc changes the morphology of the obtained nanoparticles. The materials were studied in the photodegradation of methylene blue observing that materials calcined at lower temperatures (TZ1-TZ3) have higher photocatalytic activity than those of the materials calcined at 600 degrees C (TZ4-TZ6), rutile-based systems TZ7-TZ9 are not active. Based on the background literature of titanium and zinc based nanostructures in therapeutic angiogenesis, we have explored the pro-angiogenic properties of these materials using various in vitro and in vivo assays. The zinc-doped titanium dioxide nanostructures (TZ5 and TZ6) exhibited increased cell viability, proliferation, enhanced S-phase cell population, increased pro-angiogenic messengers (ROS: reactive oxygen species and NO: nitric oxide) production and promoted in vivo blood vessel formation in a plausible mechanistic p38/STAT3 dependent signaling cascade. Altogether, the results of the present study showcase these zinc doped-titanium oxide nanoparticles as promising candidates for environmental (water-remediation) and therapeutic angiogenic applications.
Synthesis and in vitro anticancer evaluation of some 4,6-diamino-1,3,5-triazine-2-carbohydrazides as Rad6 ubiquitin conjugating enzyme inhibitors.[Pubmed:26965855]
Bioorg Med Chem Lett. 2016 Apr 15;26(8):2030-4.
Series of 4-amino-6-(arylamino)-1,3,5-triazine-2-carbohydrazides (3a-e) and N'-phenyl-4,6-bis(arylamino)-1,3,5-triazine-2-carbohydrazides (6a-e), for ease of readership, we will abbreviate our compound names as 'new triazines', have been synthesized, based on the previously reported Rad6B-inhibitory diamino-triazinylmethyl benzoate anticancer agents TZ9 and 4-amino-N'-phenyl-6-(arylamino)-1,3,5-triazine-2-carbohydrazides. Synthesis of the target compounds was readily accomplished in two steps from either bis-aryl/aryl biguanides via reaction of phenylhydrazine or hydrazinehydrate with key 4-amino-6-bis(arylamino)/(arylamino)-1,3,5-triazine-2-carboxylate intermediates. These new triazine derivatives were evaluated for their abilities to inhibit Rad6B ubiquitin conjugation and in vitro anticancer activity against several human cancer cell lines: ovarian (OV90 and A2780), lung (H1299 and A549), breast (MCF-7 and MDA-MB231) and colon (HT29) cancer cells by MTS assays. All the 10 new triazines exhibited superior Rad6B inhibitory activities in comparison to selective Rad6 inhibitor TZ9 that was reported previously. Similarly, new triazines also showed better IC50 values in survival assays of various tumor cell lines. Particularly, new triazines 6a-c, exhibited lower IC50 (3.3-22 muM) values compared to TZ9.
Design, synthesis and in vitro anticancer evaluation of 4,6-diamino-1,3,5-triazine-2-carbohydrazides and -carboxamides.[Pubmed:24153206]
Bioorg Med Chem Lett. 2013 Dec 15;23(24):6886-9.
Series of substituted 4,6-diamino-1,3,5-triazine-2-carbohydrazides and -carboxamides have been synthesised, based on molecular modelling of candidate structures related to the previously reported Rad6B-inhibitory diamino-triazinylmethyl benzoate anticancer agents TZ8 and TZ9. Synthesis of the target compounds was readily accomplished in two steps from aryl biguanides via reaction of phenylhydrazine or benzylamines with key 4-amino-6-(arylamino)-1,3,5-triazine-2-carboxylate intermediates. These new triazine derivatives were tested for in vitro anticancer activity against the Rad6B expressing human breast cancer cell lines MDA-MB-231 and MCF-7. Active compounds, such as the triazinyl-carbohydrazides 3a-e, were found to exhibit low micromolar IC50 values particularly in the Rad6B-overexpressing MDA-MB-231 cell line.


