5,5'-DimethoxysecoisolariciresinolCAS# 1002106-91-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1002106-91-3 | SDF | Download SDF |
| PubChem ID | 10002343 | Appearance | Powder |
| Formula | C22H30O8 | M.Wt | 422.47 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
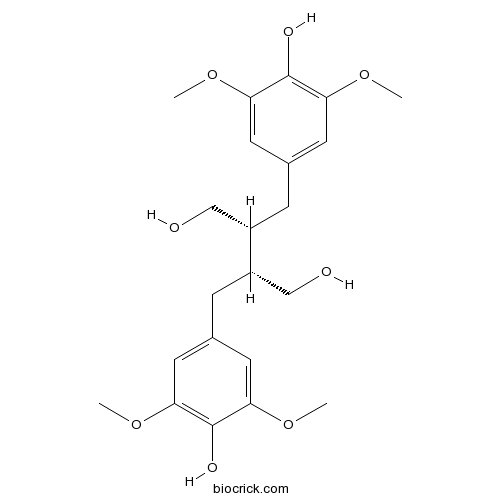
| Chemical Name | (2R,3R)-2,3-bis[(4-hydroxy-3,5-dimethoxyphenyl)methyl]butane-1,4-diol | ||
| SMILES | COC1=CC(=CC(=C1O)OC)CC(CO)C(CC2=CC(=C(C(=C2)OC)O)OC)CO | ||
| Standard InChIKey | XTRARZPRFYUCGZ-HOTGVXAUSA-N | ||
| Standard InChI | InChI=1S/C22H30O8/c1-27-17-7-13(8-18(28-2)21(17)25)5-15(11-23)16(12-24)6-14-9-19(29-3)22(26)20(10-14)30-4/h7-10,15-16,23-26H,5-6,11-12H2,1-4H3/t15-,16-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5,5'-Dimethoxysecoisolariciresinol is a natural product from Podocarpus spicata. |
| In vitro | New secoisolariciresinol derivatives from Lindera obtusiloba stems and their neuroprotective activities.[Pubmed: 19708005 ]Planta Med. 2010 Feb;76(3):294-7.
|
| Structure Identification | Zhong Yao Cai. 2015 May;38(5):972-4.Chemical Constitutes from Root of Artocarpus styracifolius.[Pubmed: 26767289]To study the chemical constituents from root of Artocarpus styracifolius. |

5,5'-Dimethoxysecoisolariciresinol Dilution Calculator

5,5'-Dimethoxysecoisolariciresinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.367 mL | 11.8352 mL | 23.6703 mL | 47.3406 mL | 59.1758 mL |
| 5 mM | 0.4734 mL | 2.367 mL | 4.7341 mL | 9.4681 mL | 11.8352 mL |
| 10 mM | 0.2367 mL | 1.1835 mL | 2.367 mL | 4.7341 mL | 5.9176 mL |
| 50 mM | 0.0473 mL | 0.2367 mL | 0.4734 mL | 0.9468 mL | 1.1835 mL |
| 100 mM | 0.0237 mL | 0.1184 mL | 0.2367 mL | 0.4734 mL | 0.5918 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2,3-dihydroxy-3-(4-hydroxyphenyl)propanoic acid
Catalog No.:BCN1641
CAS No.:100201-57-8
- Piscidinol A
Catalog No.:BCN5818
CAS No.:100198-09-2
- MK-8033
Catalog No.:BCC1768
CAS No.:1001917-37-8
- INH6
Catalog No.:BCC5455
CAS No.:1001753-24-7
- SRT1720 HCl
Catalog No.:BCC2222
CAS No.:1001645-58-4
- BV6
Catalog No.:BCC5372
CAS No.:1001600-56-1
- 1,4,5,6-Tetrahydroxy-7-prenylxanthone
Catalog No.:BCN1642
CAS No.:1001424-68-5
- PDK1 inhibitor
Catalog No.:BCC1843
CAS No.:1001409-50-2
- VUF 8430 dihydrobromide
Catalog No.:BCC7384
CAS No.:100130-32-3
- GDC-0068 (RG7440)
Catalog No.:BCC1271
CAS No.:1001264-89-6
- GnRH Associated Peptide (GAP) (1-13), human
Catalog No.:BCC1013
CAS No.:100111-07-7
- Chloramultilide D
Catalog No.:BCN7102
CAS No.:1000995-49-2
- Camstatin
Catalog No.:BCC5690
CAS No.:1002295-95-5
- AMG-208
Catalog No.:BCC1054
CAS No.:1002304-34-8
- Picrasidine I
Catalog No.:BCN5819
CAS No.:100234-59-1
- Picrasidine J
Catalog No.:BCN5820
CAS No.:100234-62-6
- TZ9
Catalog No.:BCC5547
CAS No.:1002789-86-7
- Irinotecan hydrochloride
Catalog No.:BCN2949
CAS No.:100286-90-6
- Apiopaeonoside
Catalog No.:BCN2801
CAS No.:100291-86-9
- Pemirolast potassium
Catalog No.:BCC4532
CAS No.:100299-08-9
- L(+)-Rhamnose monohydrate
Catalog No.:BCN8368
CAS No.:10030-85-0
- Curcumenone
Catalog No.:BCN3008
CAS No.:100347-96-4
- Calcium chloride dihydrate
Catalog No.:BCC7582
CAS No.:10035-04-8
- GSK 1562590 hydrochloride
Catalog No.:BCC8010
CAS No.:1003878-07-6
New secoisolariciresinol derivatives from Lindera obtusiloba stems and their neuroprotective activities.[Pubmed:19708005]
Planta Med. 2010 Feb;76(3):294-7.
A methanolic extract of Lindera obtusiloba (Lauraceae) stems significantly attenuated glutamate-induced oxidative stress in HT22 hippocampal cells. Two new secoisolariciresinol derivatives, characterized as 9,9'- O-di-(E)-feruloyl-meso-5,5'-dimethoxysecoisolariciresinol (1) and 9,9'-O-di-(E)-sinapinoyl-meso-5,5'-dimethoxysecoisolariciresinol (2), were isolated, along with the known compound 9,9'-O-di-(E)-feruloyl-meso-secoisolariciresinol (3), from the methanolic extract of L. obtusiloba stems. Compounds 1, 2, and 3 showed neuroprotective effects on glutamate-induced toxicity in HT22 cells.
[Chemical Constitutes from Root of Artocarpus styracifolius].[Pubmed:26767289]
Zhong Yao Cai. 2015 May;38(5):972-4.
OBJECTIVE: To study the chemical constituents from root of Artocarpus styracifolius. METHODS: Tne constituents were isolated from the root of Artocarpus styracifolius by column chromatography over silica gel, RP-18 silica gel, MCI GEL CHP-20P, macroporous resin HP-20, Sephadex LH-20, Toyopearl HW-40C and by preparative HPLC. Their structures were elucidated by analysis of physical and chemical properties and spectral data. RESULTS: Nine compounds were isolated and their structures were identified as p-hydroxy benzoic acid (1), syringic acid (2), 2,4-dihydroxy benzaldehyde (3), (+)-lyoniresinol (4), 5,5'-dimethoxysecoisolariciresinol (5), (+)- syringaresinol (6), scopoletin (7), xylarolide (8) and trans-oxyresveratrol (9). CONCLUSION: Compounds 2, 5, 6 and 8 are isolated from Moraceae for the first time. Compounds 1, 4 and 7 are firstly characterized in the genus Artocarpus, compounds 3 and 9 are characterized in Artocarpus styracifolius for the first time.


