1,4-CineoleCAS# 470-67-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 470-67-7 | SDF | File under preparation. |
| PubChem ID | 10106 | Appearance | Oil |
| Formula | C10H18O | M.Wt | 154.2 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
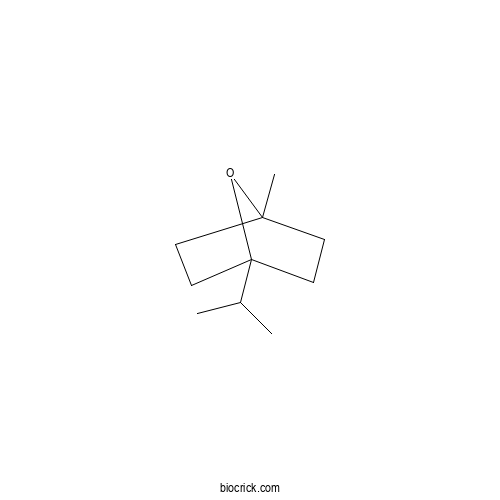
| Chemical Name | 1-methyl-4-propan-2-yl-7-oxabicyclo[2.2.1]heptane | ||
| SMILES | CC(C)C12CCC(O1)(CC2)C | ||
| Standard InChIKey | RFFOTVCVTJUTAD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H18O/c1-8(2)10-6-4-9(3,11-10)5-7-10/h8H,4-7H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1,4-Cineole presents potential anxiolytic-like action consistent with possible general depression of the CNS. | |||||

1,4-Cineole Dilution Calculator

1,4-Cineole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4851 mL | 32.4254 mL | 64.8508 mL | 129.7017 mL | 162.1271 mL |
| 5 mM | 1.297 mL | 6.4851 mL | 12.9702 mL | 25.9403 mL | 32.4254 mL |
| 10 mM | 0.6485 mL | 3.2425 mL | 6.4851 mL | 12.9702 mL | 16.2127 mL |
| 50 mM | 0.1297 mL | 0.6485 mL | 1.297 mL | 2.594 mL | 3.2425 mL |
| 100 mM | 0.0649 mL | 0.3243 mL | 0.6485 mL | 1.297 mL | 1.6213 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Delta-Nonalactone
Catalog No.:BCN0065
CAS No.:3301-94-8
- Kaempferol 3,4'-diglucoside 7-rhamnoside
Catalog No.:BCN0064
CAS No.:1131009-93-2
- Acetic acid m-cresyl ester
Catalog No.:BCN0063
CAS No.:122-46-3
- 2',6'-Dihydroxy-4,4'-dimethoxychalcone
Catalog No.:BCN0062
CAS No.:20621-49-2
- 4-Isopropylbenzaldehyde
Catalog No.:BCN0061
CAS No.:122-03-2
- Dihydroxyfumaric acid
Catalog No.:BCN0060
CAS No.:133-38-0
- Apigenin 4'-O-(2'',6''-di-O-E-p-coumaroyl)glucoside
Catalog No.:BCN0059
CAS No.:71781-79-8
- Myosmine
Catalog No.:BCN0058
CAS No.:532-12-7
- Hippuric acid
Catalog No.:BCN0057
CAS No.:495-69-2
- 3-Ethoxy-4-hydroxybenzaldehyde
Catalog No.:BCN0056
CAS No.:121-32-4
- 2-Nonyl alcohol
Catalog No.:BCN0055
CAS No.:628-99-9
- 6-Hydroxyflavanone
Catalog No.:BCN0054
CAS No.:4250-77-5
- (R)-(+)-Limonene
Catalog No.:BCN0067
CAS No.:5989-27-5
- Undecanolactone
Catalog No.:BCN0068
CAS No.:710-04-3
- Laricitrin
Catalog No.:BCN0069
CAS No.:53472-37-0
- Peucenidin
Catalog No.:BCN0070
CAS No.:33044-93-8
- Homobutein
Catalog No.:BCN0071
CAS No.:34000-39-0
- Undecanoic gamma-lactone
Catalog No.:BCN0072
CAS No.:104-67-6
- Procyanidin B4
Catalog No.:BCN0073
CAS No.:29106-51-2
- Gitoxin
Catalog No.:BCN0074
CAS No.:4562-36-1
- Isoarundinin II
Catalog No.:BCN0075
CAS No.:151538-56-6
- 3,4-Dihydroxy-5-methoxybenzoic acid
Catalog No.:BCN0076
CAS No.:3934-84-7
- Cimiaceroside A
Catalog No.:BCN0077
CAS No.:210643-83-7
- 2-Octanone
Catalog No.:BCN0078
CAS No.:111-13-7
Sedative and hypnotic effects of Perilla frutescens essential oil through GABAergic system pathway.[Pubmed:33246117]
J Ethnopharmacol. 2020 Nov 24:113627.
ETHNOPHARMACOLOGICAL RELEVANCE: Traditional Chinese medicine believes that depression syndrome has become one of the core pathogenesis of insomnia. The pharmacology of traditional Chinese medicine points out that Perilla frutescens has the effect of regulating Qi and relieving depression, promoting Qi circulation to relieve pain, so Perilla frutescens may have the potential therapeutic effect on insomnia. Related studies have reported the sedative and hypnotic effects of Perilla frutescens, but these studies have not yet explored the mechanism of sedative and hypnotic effects of Perilla frutescens essential oil (PFEO) through inhalation administration. AIM OF THE STUDY: The purpose of this study is to explore the underlying sedative and hypnotic mechanisms of PFEO through the GABAergic system pathways. MATERIALS AND METHODS: Established the PCPA insomnia model of mice, The open field test, pentobarbital-induced falling asleep rate, latency of sleeping time, and duration of sleeping time experiments were used to evaluate the behavior of mice, the enzyme-linked immunosorbent assay was used to analyze the content of 5-HT and GABA in hypothalamus and cerebral cortex. Immunohistochemical experiment, Western blot experiment and RT-PCR experiment were used to study the mechanism of PFEO through GABAergic pathway to regulate insomnia. The main volatile constituents of PFEO were analyzed by gas chromatography-mass spectrometry (GC-MS). RESULTS: The inhalation of PFEO has sedative and hypnotic effects, which reduce significantly the autonomic activity of PCPA insomnia mice, increase falling asleep rate, shorten latency of sleeping time, and prolong duration of sleeping time; the results of enzyme-linked immunosorbent assay show that PFEO increase the content of 5-HT and GABA in hypothalamus and cerebral cortex. The results showed that inhalation of PFEO increase the expression of GABAAalpha1 and GABAAalpha2 positive cells, increase the level of GABAAalpha1 and GABAAalpha2 protein and also increase the level of GABAAalpha1 mRNA and GABAAalpha2 mRNA in the hypothalamus and cerebral cortex. The highest content of PFEO is Perillaldehyde (54.37%), followed by 1,4-Cineole (7.42%), Acetaldehyde diethyl acetal (6.61%), D-Limonene (5.09%), Eucalyptol (4.94%), etc. CONCLUSION: The inhalation of PFEO has sedative and hypnotic effects, it is speculated that the mechanism of which may be the sedative and hypnotic effects through the GABAergic pathway.
Monoterpenes as therapeutic candidates to induce fetal hemoglobin synthesis and up-regulation of gamma-globin gene: An in vitro and in vivo investigation.[Pubmed:33137331]
Eur J Pharmacol. 2020 Nov 1:173700.
Pharmacologically induced production of fetal hemoglobin (HbF) is a pragmatic therapeutic strategy for the reduction of globin chain imbalance and improving the clinical severities of patients with beta-hemoglobinopathies. To identify highly desirable new therapeutic HbF-inducing agents, we screened functionally diverse ten monoterpenes, as molecular entities for their potent induction and erythroid differentiation ability in human erythroleukemia cell line (K562) and transgenic mice. Benzidine hemoglobin staining demonstrated six compounds to have significantly induced erythroid differentiation of K562 cells in a dose and time-dependent manner. This induction paralleled well with the optimal accumulated quantity of total hemoglobin in treated cultures. The cytotoxic studies revealed that three (carvacrol, 3-carene, and 1,4-Cineole) of the six compounds with their maximal erythroid expansion ability did not affect cell proliferation and were found non-toxic. Four compounds were found to have high potency, with 4-8-fold induction of HbF at both transcriptional and protein levels in vitro. Subsequently, an in vivo study with the three active non-cytotoxic compounds showed significant overexpression of the gamma-globin gene and HbF production. Carvacrol emerged as a lead HbF regulator suggested by the increase in expression of gamma-globin mRNA content (5.762 +/- 0.54-fold in K562 cells and 5.59 +/- 0.20-fold increase in transgenic mice), accompanied by an increase in fetal hemoglobin (F-cells) levels (83.47% in K562 cells and 79.6% in mice model). This study implicates monoterpenes as new HbF inducing candidates but warrants mechanistic elucidation to develop them into potential therapeutic drugs in beta-thalassemia and sickle cell anemia.
Inhalation Administration of the Bicyclic Ethers 1,8- and 1,4-cineole Prevent Anxiety and Depressive-Like Behaviours in Mice.[Pubmed:32325759]
Molecules. 2020 Apr 18;25(8). pii: molecules25081884.
The anxiolytic and antidepressant-like activities of the naturally occurring monoterpene 1,8-cineole and its structural isomer 1,4-Cineole were evaluated in mice via inhalation administration at doses ranging from 4 x 10(-6) to 4 x 10(-1) mg per 400 muL of triethyl citrate. Mice were tested for anxiety-like behaviours by using the light-dark box test (LDB) and marble-burying test (MBT) and for depression-like symptoms by using the forced swimming test (FST) and tail suspension test (TST). Diazepam and fluoxetine were used as standard drugs for anxiolytic and antidepressant tests, respectively. The results showed that 1,8-cineole at 4 x 10(-4) mg, and 1,4-Cineole at 4 x 10(-4) and 4 x 10(-3) mg significantly increased the amount of time spent in the light box and the number of entries in the light box in the LDB as well as reduced the number of marbles buried in the MBT relative to those in the control, suggesting an anxiolytic effect. Similarly, 1,8-cineole at 4 x 10(-4) and 4 x 10(-2) mg and 1,4-Cineole at doses of 4 x 10(-4) to 4 x 10(-2) mg significantly reduced immobility times in the FST and TST relative to those of the control, suggesting an antidepressant activity. The role of the GABAA/benzodiazepine receptor system in the anxiolytic effects of 1,8- and 1,4-Cineole was investigated through co-administration of flumazenil, a GABAergic system antagonist. Flumazenil reversed the effects of diazepam and 1,8-cineole, suggesting that 1,8-cineole affects the GABAA/benzodiazepine receptors. Collectively, the results suggest that inhaled 1,8- and 1,4-Cineole prevented anxiety and depressive-like symptoms in classic mice models.
Changes in Red Wine Composition during Bottle Aging: Impacts of Grape Variety, Vineyard Location, Maturity, and Oxygen Availability during Aging.[Pubmed:32066244]
J Agric Food Chem. 2020 Nov 25;68(47):13331-13343.
This work investigated the influence of grape variety, vineyard location, and grape harvest maturity, combined with different oxygen availability treatments, on red wine composition during bottle aging. Chemometric analysis of wine compositional data (i.e., wine color parameters, SO2, metals, and volatile compounds) demonstrated that the wine samples could be differentiated according to the different viticultural or bottle-aging factors. Grape variety, vineyard location, and grape maturity showed greater influence on wine composition than bottle-aging conditions. For most measured wine compositional variables, the evolution patterns adopted from the viticultural factors were not altered by oxygen availability treatment. However, contrasting evolution patterns for some variables were observed according to specific viticultural factors, with examples including dimethyl sulfide, phenylacetaldehyde, maltol, and beta-damascenone for vineyard locations, 2-methylbutanal, 1,4-Cineole, and linalool for grape variety, and methanethiol, methional, and homofuraneol for grape maturity.
Terpenes and Phenylpropanoids as Acetyl- and Butyrylcholinesterase Inhibitors: A Comparative Study.[Pubmed:31660828]
Curr Alzheimer Res. 2019;16(10):963-973.
BACKGROUND: Cholinesterase inhibitors are routinely applied in the treatment of Alzheimer's disease, and seeking new cholinesterase inhibitors is a priority. OBJECTIVES: Twenty seven compounds were compared, including ones not previously tested. An attempt was undertaken to precisely describe the role of alcohol in the inhibitory activity. This paper underlines the role of a "false positive" blank sample in the routine analysis. METHODS: The inhibition of cholinesterase was measured using Ellman's colorimetric method with a few modifications designed by the authors (including the "false-positive" effect). The inhibitory role of ethanol and methanol was also carefully evaluated. The present and past results were compared taking the source of enzyme and alcohol content into consideration. RESULTS: For the first time, new inhibitors were identified, namely: methyl jasmonate, 1R-(-)-nopol ((anti-acetyl-(AChE) and butyrylcholinesterase (BChE) activity)) and 1,4-Cineole, allo-aromadendrene, nerolidol, beta-ionone, and (R)-(+)-pulegone (anti-BChE activity). Oleanolic acid and (+)-beta-citronellene (not previously studied) proved to be inefficient inhibitors. For a number of well-known inhibitors (such as nerol, (-)-menthol, (+)-menthol, isoborneol, (-)-bornyl acetate, limonene, alpha-pinene, beta-pinene, alpha- ionone, and eugenol) some serious discrepancies were observed between our findings and the results of previous studies. Ethanol and methanol showed no anti-AChE activity up to 0.29% (v/v) and 0.23% (v/v), respectively. Similarly, ethanol up to 0.33% (v/v) and methanol up to 0.29% (v/v) did not inhibit the activity of BChE. CONCLUSION: It can be stated that the impact of alcohol should be precisely determined and that blank "false-positive" samples should be processed together with test samples. Furthermore, the effect of the enzyme origin on the result of this test must be taken into consideration.
Selective hydroxylation of 1,8- and 1,4-cineole using bacterial P450 variants.[Pubmed:30590022]
Arch Biochem Biophys. 2019 Mar 15;663:54-63.
This study has evaluated the use of the P450 metalloenzymes CYP176A1, CYP101A1 and CYP102A1, together with engineered protein variants of CYP101A1 and CYP102A1, to alter the regioselectivity of 1,8- and 1,4-Cineole hydroxylation. CYP176A1 was less selective for 1,4-Cineole oxidation when compared to its preferred substrate, 1,8-cineole. The CYP102A1 variants significantly improved the activity over the WT enzyme for oxidation of 1,4- and 1,8-cineole. The CYP102A1 R47L/Y51F/A74G/F87V/L188Q mutant generated predominantly (1S)-6alpha-hydroxy-1,8-cineole (78% e.e.) from 1,8-cineole. Oxidation of 1,4-Cineole by the CYP102A1 R47L/Y51F/F87A/I401P variant generated the 3alpha product in >90% yield. WT CYP101A1 formed a mixture metabolites with 1,8-cineole and very little product was generated with 1,4-Cineole. In contrast the F87W/Y96F/L244A/V247L and F87W/Y96F/L244A variants of CYP101A1 favoured formation of 5alpha-hydroxy-1,8-cineole (>88%, 1S 86% e.e.) while the F87V/Y96F/L244A variant generated (1S)-6alpha-hydroxy-1,8-cineole in excess (90% regioselective, >99% e.e.). The CYP101A1 F87W/Y96F/L244A/V247L and F87W/Y96F/L244A mutants improved the oxidation of 1,4-Cineole generating an excess of the 3alpha metabolite (1S>99% e.e. with the latter). The CYP101A1 F87L/Y96F variant also improved the oxidation of this substrate but shifted the site of oxidation to the isopropyl group, (8-hydroxy-1,4-Cineole). When this 8-hydroxy metabolite was generated in significant quantities desaturation of C8C9 to the corresponding alkene was also detected.
Norisoprenoids, Sesquiterpenes and Terpenoids Content of Valpolicella Wines During Aging: Investigating Aroma Potential in Relationship to Evolution of Tobacco and Balsamic Aroma in Aged Wine.[Pubmed:29616214]
Front Chem. 2018 Mar 19;6:66.
During wine aging, tobacco and balsamic aroma notes appear. In this paper, volatile compounds directly or potentially related to those aromas have been investigated in Corvina and Corvinone wines during aging. Corvina and Corvinone are two northern-Italy autochthonous red grape varieties, used to produce Valpolicella Classico and Amarone wines, both characterized by tobacco and balsamic aroma notes. Wines were analyzed shortly after bottling or following model aging at 60 degrees C for 48, 72, and 168 h. Volatile compounds were analyzed by HS-SPME-GC-MS. Results showed that compounds related to tobacco aroma [beta-damascenone, 3-oxo-alpha-ionol, (E)-1-(2,3,6-Trimethylphenyl)-buta-1,3-diene (TPB), and megastigmatrienones] increased in relationship to storage time with different patterns. beta-Damascenone and 3-oxo-alpha-ionol rapidly increased to reach a plateau in the first 48-72 h of model aging. Instead, TPB and megastigmatrienones concentration showed a linear correlation with aging time. During model aging, several cyclic terpenes tended to increase. Among them 1,8-cineole and 1,4-Cineole, previously reported to contribute to red wine eucalyptus notes increased proportionally to storage time, and this behavior was clearly associated with reactions involving alpha-terpineol, limonene, and terpinolene, as confirmed by studies with model wine solutions. Among other relevant volatile compounds, sesquiterpenes appear to contribute potentially balsamic and spicy aroma notes. In this study, linear sesquiterpenes (nerolidol, farnesol) underwent acid hydrolysis during long wine aging, while cyclic sesquiterpenes seemed to increase with time. The chemical pathways associated with evolution of some of the compounds investigated have been studied in model wine.
Mechanism of ascaridole activation in Leishmania.[Pubmed:28263719]
Biochem Pharmacol. 2017 May 15;132:48-62.
Endoperoxides (EP) are an emerging class of drugs which have potential in antiparasitic therapy, but also in other fields. For malaria therapy the EP artemisinin (Art) and its derivatives are successfully used. We have shown in the past that the EP ascaridole (Asc) is useful for the treatment of cutaneous leishmaniasis in a mouse model. Biomimetic experiments suggested that these drugs need activation in the respective target pathogens to exert their function. In spite of this idea, direct activation of EP to radicals inside cells has never been demonstrated. Therefore, this study was initiated to explore the activation of Asc in biomimetic systems and inside Leishmania in comparison to Art. Using electron paramagnetic resonance spectroscopy (EPR) in combination with spin-trapping we identified the secondary alkyl radical intermediates arising from reduction by Fe(2+) in cell-free systems. Combined GC/NMR analysis confirmed the loss of isopropyl residues from Asc during this process as intermediates. This activation of Asc was stimulated by low molecular Fe(2+) complexes or alternatively by hemin in conjunction with thiol reductants, such as cysteine (Cys). In Leishmania tarentolae promastigotes (LtP) as model for pathogenic forms of Leishmania carbon-centered radicals were identified in the presence of Asc by EPR spin-trapping. Both Asc and Art inhibited the viability in LtP with IC50 values in the low micromolar range while IC50 values for J774 macrophages were considerably higher. A similar structure without EP bridge (1,4-Cineole) resulted in no detectable radicals and possessed much less cytotoxicity in LtP and no selectivity for LtP compared to J774 cells. The Asc-derived radical formation in LtP was inhibited by the iron chelator deferoxamine (DFO), and stimulated by Cys (a suitable reductant for hemin). The IC50 values for LtP viability in the presence of Asc or Art were increased significantly by the spin trap DMPO, while Cys and DFO increased only IC50 values for Art. In a heme association assay Asc demonstrated a lower binding affinity to heme than Art. ICP-OES measurements revealed that in LtP the total iron concentrations were twice as high as values in J774 macrophages. Since low molecular iron was important in Asc activation we studied the influence of Asc on the labile iron pool (LIP) in LtP. Low temperature EPR experiments demonstrated that Asc shifts the redox balance of iron in the LIP to its oxidized state. These data demonstrate that univalent cleavage of Asc/Art in LtP is an essential part of their pharmacological mechanism. The structure of the EP determines whether activation by low molecular iron or heme is favored and the availability of these intracellular activators modulates their cytotoxicity. These findings may be helpful for synthesis of new Asc derivatives and understanding the action of EP in other cell types.
Interaction of plant essential oil terpenoids with the southern cattle tick tyramine receptor: A potential biopesticide target.[Pubmed:27986436]
Chem Biol Interact. 2017 Feb 1;263:1-6.
An outbreak of the southern cattle tick, Rhipicephalus (Boophilus) microplus, (Canestrini), in the United States would have devastating consequences on the cattle industry. Tick populations have developed resistance to current acaricides, highlighting the need to identify new biochemical targets along with new chemistry. Furthermore, acaricide resistance could further hamper control of tick populations during an outbreak. Botanically-based compounds may provide a safe alternative for efficacious control of the southern cattle tick. We have developed a heterologous expression system that stably expresses the cattle tick's tyramine receptor with a G-protein chimera, producing a system that is amenable to high-throughput screening. Screening an in-house terpenoid library, at two screening concentrations (10 muM and 100 muM), has identified four terpenoids (piperonyl alcohol, 1,4-Cineole, carvacrol and isoeugenol) that we believe are positive modulators of the southern cattle tick's tyramine receptor.
Differential Activation of TRP Channels in the Adult Rat Spinal Substantia Gelatinosa by Stereoisomers of Plant-Derived Chemicals.[Pubmed:27483289]
Pharmaceuticals (Basel). 2016 Jul 28;9(3). pii: ph9030046.
Activation of TRPV1, TRPA1 or TRPM8 channel expressed in the central terminal of dorsal root ganglion (DRG) neuron increases the spontaneous release of l-glutamate onto spinal dorsal horn lamina II (substantia gelatinosa; SG) neurons which play a pivotal role in regulating nociceptive transmission. The TRP channels are activated by various plant-derived chemicals. Although stereoisomers activate or modulate ion channels in a distinct manner, this phenomenon is not fully addressed for TRP channels. By applying the whole-cell patch-clamp technique to SG neurons of adult rat spinal cord slices, we found out that all of plant-derived chemicals, carvacrol, thymol, carvone and cineole, increase the frequency of spontaneous excitatory postsynaptic current, a measure of the spontaneous release of l-glutamate from nerve terminals, by activating TRP channels. The presynaptic activities were different between stereoisomers (carvacrol and thymol; (-)-carvone and (+)-carvone; 1,8-cineole and 1,4-Cineole) in the extent or the types of TRP channels activated, indicating that TRP channels in the SG are activated by stereoisomers in a distinct manner. This result could serve to know the properties of the central terminal TRP channels that are targets of drugs for alleviating pain.
In vitro Screening of Essential Oil Active Compounds for Manipulation of Rumen Fermentation and Methane Mitigation.[Pubmed:26954157]
Asian-Australas J Anim Sci. 2016 Jul;29(7):952-9.
The objective of this study was to investigate the effects of 11 active compounds of essential oils (ACEO) on rumen fermentation characteristics and methane production. Two trials were conducted. In trial 1, ACEO (eugenol, carvacrol, citral, limonene, 1,4-Cineole, p-cymene, linalool, bornyl acetate, alpha-pinene, and beta-pinene) at a dose of 1,000 muL/L were incubated for 24 h in diluted rumen fluid with a 70:30 forage:concentrate substrate (16.2% crude protein; 36.6% neutral detergent fiber). Three fistulated Holstein cows were used as donors of rumen fluid. The reduction in methane production was observed with nine ACEO (up to 86% reduction) compared with the control (p<0.05). Among these, only limonene, 1,4-Cineole, bornyl acetate, and alpha-pinene did not inhibit volatile fatty acid (VFA) production, and only bornyl acetate produced less methane per mol of VFA compared with the control (p<0.05). In a subsequent trial, the effects on rumen fermentation and methane production of two concentrations (500 and 2,000 muL/L) of bornyl acetate, the most promising ACEO from the first trial, were evaluated using the same in vitro incubation method that was used in the first trial. In trial 2, monensin was used as a positive control. Both doses of bornyl acetate decreased (p<0.05) methane production and did not inhibit VFA production. Positive effects of bornyl acetate on methane and VFA production were more pronounced than the effects of monensin. These results confirm the ability of bornyl acetate to decrease methane production, which may help to improve the efficiency of energy use in the rumen.
1,8- and 1,4-cineole enhance spontaneous excitatory transmission by activating different types of transient receptor potential channels in the rat spinal substantia gelatinosa.[Pubmed:26578070]
J Neurochem. 2016 Feb;136(4):764-777.
Although transient receptor potential (TRP) channels expressed in the spinal substantia gelatinosa play a role in modulating nociceptive transmission, their properties have not been fully examined yet. In order to address this issue, the effects of 1,8-cineole and its stereoisomer 1,4-Cineole on excitatory transmission were examined by applying the whole-cell patch-clamp technique to substantia gelatinosa neurons in adult rat spinal cord slices. Miniature excitatory postsynaptic current frequency was increased by 1,8- and 1,4-Cineole. The cineole activities were repeated and resistant to voltage-gated Na(+) -channel blocker tetrodotoxin. The 1,8-cineole activity was inhibited by TRP ankyrin-1 (TRPA1) antagonists (HC-030031 and mecamylamine) but not TRP vanilloid-1 (TRPV1) antagonists (capsazepine and SB-366791), whereas the 1,4-Cineole activity was depressed by the TRPV1 but not TRPA1 antagonists. Although 1,8- and 1,4-Cineole reportedly activate TRP melastatin-8 (TRPM8) channels, their activities were unaffected by TRPM8 antagonist 4-(3-chloro-2-pyridinyl)-N-[4-(1,1-dimethylethyl)phenyl]-1-piperazinecarboxamide. Monosynaptically evoked C-fiber, but not Adelta-fiber excitatory postsynaptic current amplitude, was reduced by 1,8- and 1,4-Cineole. These results indicate that 1,8- and 1,4-Cineole increase spontaneous l-glutamate release from nerve terminals by activating TRPA1 and TRPV1 channels, respectively, while inhibiting C-fiber but not Adelta-fiber evoked l-glutamate release. This difference between 1,8- and 1,4-Cineole may serve to know the properties of TRP channels located in the central terminals of primary-afferent neurons. The spinal dorsal horn lamina II (substantia gelatinosa; SG) plays a pivotal role in regulating nociceptive transmission from the periphery. We found out in the SG that 1,4- and 1,8-cineole activate TRPV1 and TRPA1 channels, respectively, located in primary-afferent, possibly C-fiber, central terminals. This difference may serve to know the properties of TRP channels expressed in the central terminals.
Investigation and Sensory Characterization of 1,4-Cineole: A Potential Aromatic Marker of Australian Cabernet Sauvignon Wine.[Pubmed:26434979]
J Agric Food Chem. 2015 Oct 21;63(41):9103-11.
This work reports the quantitation and sensory characterization of 1,4-Cineole in red wine for the first time. A headspace-solid-phase microextraction-gas chromatography-mass spectrometry (HS-SPME-GC-MS) method was developed to quantitate 1,4-Cineole and 1,8-cineole in 104 commercial Australian red wines. 1,4-Cineole was detected in all of the wines analyzed, with concentrations ranging from 0.023 to 1.6 mug/L. An important varietal effect was observed, with concentrations of 1,4-Cineole in Cabernet Sauvignon wines (mean of 0.6 +/- 0.3 mug/L) significantly higher than in Shiraz (0.07 +/- 0.04 mug/L) and Pinot Noir (0.2 +/- 0.2 mug/L) wines. Regional variations of both cineole isomer concentrations have been measured between wines originating from different Australian regions. Sensory studies demonstrated that the addition of 0.54 mug/L 1,4-Cineole in a Cabernet Sauvignon wine, to produce a final concentration of 0.63 mug/L, was perceived significantly by a sensory panel (p < 0.05). Descriptive analyses revealed that 1,4-Cineole and 1,8-cineole may contribute to the hay, dried herbs, and blackcurrant aromas reported in Australian Cabernet Sauvignon wines and may be potential markers of regional typicality of these wines.


