1-IsomangostinCAS# 19275-44-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19275-44-6 | SDF | Download SDF |
| PubChem ID | 5281641 | Appearance | Powder |
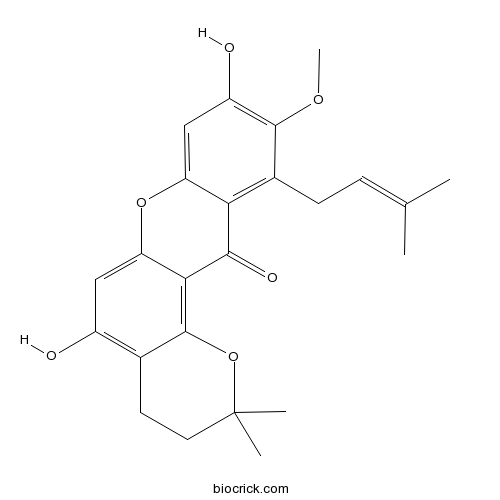
| Formula | C24H26O6 | M.Wt | 410.5 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5,9-dihydroxy-10-methoxy-2,2-dimethyl-11-(3-methylbut-2-enyl)-3,4-dihydropyrano[2,3-a]xanthen-12-one | ||
| SMILES | CC(=CCC1=C2C(=CC(=C1OC)O)OC3=CC(=C4CCC(OC4=C3C2=O)(C)C)O)C | ||
| Standard InChIKey | JUHXHWKPHWGZKL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H26O6/c1-12(2)6-7-14-19-17(11-16(26)22(14)28-5)29-18-10-15(25)13-8-9-24(3,4)30-23(13)20(18)21(19)27/h6,10-11,25-26H,7-9H2,1-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1-Isomangostin has cytotoxic, and anticomplement activities. |
| Targets | p65 | NF-kB |
| In vitro | A new xanthone from the pericarp of Garcinia mangostana.[Pubmed: 24555285]Nat Prod Commun. 2013 Dec;8(12):1733-4.
|
| Cell Research | Cytotoxic xanthone constituents of the stem bark of Garcinia mangostana (mangosteen).[Pubmed: 19839614 ]J Nat Prod. 2009 Nov;72(11):2028-31
|
| Structure Identification | Phytother Res. 2010 Oct;24(10):1575-7.Xanthone constituents of the fruits of Garcinia mangostana with anticomplement activity.[Pubmed: 20878711]
|

1-Isomangostin Dilution Calculator

1-Isomangostin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4361 mL | 12.1803 mL | 24.3605 mL | 48.7211 mL | 60.9013 mL |
| 5 mM | 0.4872 mL | 2.4361 mL | 4.8721 mL | 9.7442 mL | 12.1803 mL |
| 10 mM | 0.2436 mL | 1.218 mL | 2.4361 mL | 4.8721 mL | 6.0901 mL |
| 50 mM | 0.0487 mL | 0.2436 mL | 0.4872 mL | 0.9744 mL | 1.218 mL |
| 100 mM | 0.0244 mL | 0.1218 mL | 0.2436 mL | 0.4872 mL | 0.609 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Indole-3-acetic acid
Catalog No.:BCN8716
CAS No.:87-51-4
- 2-O-Acetyl-20-hydroxyecdysone
Catalog No.:BCN8715
CAS No.:19536-25-5
- Glycinol
Catalog No.:BCN8714
CAS No.:69393-95-9
- Bryonamide A
Catalog No.:BCN8713
CAS No.:75268-14-3
- Methyl caffeate acid
Catalog No.:BCN8712
CAS No.:3843-74-1
- Quercetin 3-O-beta-(6''-p-coumaroyl)glucopyranosyl(1->2)-alpha-L-rhamnopyranoside
Catalog No.:BCN8711
CAS No.:143061-65-8
- Asiaticoside B
Catalog No.:BCN8709
CAS No.:125265-68-1
- Isocarlinoside
Catalog No.:BCN8708
CAS No.:83151-90-0
- Pilosidine
Catalog No.:BCN8707
CAS No.:229971-57-7
- Trikamsteroside E
Catalog No.:BCN8706
CAS No.:952579-37-2
- 5,6,7,4'-tetrahydroxyisoflavone-6,7-di-O-beta-D-glucopyranoside
Catalog No.:BCN8705
CAS No.:1219001-04-3
- Xanthoangelol B
Catalog No.:BCN8704
CAS No.:132998-81-3
- Nagarine
Catalog No.:BCN8718
CAS No.:41849-35-8
- 3-Methylxanthine
Catalog No.:BCN8719
CAS No.:1076-22-8
- 19 alpha-Hydroxyasiatic acid
Catalog No.:BCN8720
CAS No.:70868-78-9
- Dihydrowithaferin A
Catalog No.:BCN8721
CAS No.:5589-41-3
- Oleuroside
Catalog No.:BCN8722
CAS No.:116383-31-4
- Trikamsteroside C
Catalog No.:BCN8724
CAS No.:952579-35-0
- Pseudolaric acid C2
Catalog No.:BCN8726
CAS No.:82508-35-8
- 3-O-Acetyl-20-hydroxyecdysone
Catalog No.:BCN8728
CAS No.:22961-68-8
- N-Benzyloctadecanamide
Catalog No.:BCN8725
CAS No.:5327-45-7
- Hamaudol
Catalog No.:BCN6371
CAS No.:735-46-6
- Celosin I
Catalog No.:BCN8723
CAS No.:1807732-38-2
- Polygalin C
Catalog No.:BCN8738
CAS No.:934768-05-5
A new xanthone from the pericarp of Garcinia mangostana.[Pubmed:24555285]
Nat Prod Commun. 2013 Dec;8(12):1733-4.
A new prenylxanthone, garcimangostanol (1), was isolated from the EtOAc-soluble partition of the ethanol extract of the pericarp of Garcinia mangostana L., along with three known compounds, namely 8-deoxygartanin (2), 1-Isomangostin (3), and garcinone C (4). The structure of compound 1 was elucidated on the basis of its 1D, 2D NMR and MS data. Compounds 1-4 exhibited either significant o r moderate cytotoxicity against MCF-7, A549, Hep-G2 and CNEhuman cancer cell lines in vitro with IC50 values from 4.0 +/- 0.3 to 23.6+/- 1.5 microM by MTT colorimetric assay.
3-O-Methyl-1-isomangostin.[Pubmed:22719700]
Acta Crystallogr Sect E Struct Rep Online. 2012 Jun 1;68(Pt 6):o1950-1.
IN THE TITLE XANTHONE DERIVATIVE [SYSTEMATIC NAME: 9-hy-droxy-5,10-dimeth-oxy-2,2-dimethyl-11-(3-methyl-but-2-en-1-yl)-2,3,4,12-tetr a-hydro-1,7-dioxatetra-phen-12-one], C(25)H(28)O(6), the xanthone ring system is roughly planar, with an r.m.s. deviation of 0.1038 (1) A. The chromane ring is in a half-chair conformation and the 3-methyl-but-2-enyl substituent is axially attached with an (+)-anti-clinal conformation. Two weak intra-molecular C-Hcdots, three dots, centeredO inter-actions generate two S(6) ring motifs. In the crystal, mol-ecules are linked into ribbons along the c axis by O-Hcdots, three dots, centeredO and weak C-Hcdots, three dots, centeredO hydrogen bonds. A pi-pi inter-action, with a centroid-centroid distance of 3.5413 (8) A, is also observed.
Xanthone constituents of the fruits of Garcinia mangostana with anticomplement activity.[Pubmed:20878711]
Phytother Res. 2010 Oct;24(10):1575-7.
Phytochemical investigation of a chloroform-soluble fraction of the freeze-dried fruits of Garcinia mangostana (Clusiaceace) with anticomplement activity in the classical pathway led to the identification of five known xanthones. The structures of these compounds were confirmed by interpretation of NMR and MS spectroscopic data. Of the isolates obtained, 1-Isomangostin and garcinone E were found to be active constituents in the anticomplement assay used.
Cytotoxic xanthone constituents of the stem bark of Garcinia mangostana (mangosteen).[Pubmed:19839614]
J Nat Prod. 2009 Nov;72(11):2028-31.
Bioassay-guided fractionation of a chloroform-soluble extract of Garcinia mangostana stem bark, using the HT-29 human colon cancer cell line and an enzyme-based ELISA NF-kappaB assay, led to the isolation of a new xanthone, 11-hydroxy-3-O-methyl-1-Isomangostin (1). The structure of 1 was elucidated by spectroscopic data analysis. In addition, 10 other known compounds, 11-hydroxy-1-Isomangostin (2), 11alpha-mangostanin (3), 3-isomangostin (4), alpha-mangostin (5), beta-mangostin (6), garcinone D (7), 9-hydroxycalabaxanthone (8), 8-deoxygartanin (9), gartanin (10), and cratoxyxanthone (11), were isolated. Compounds 4-8 exhibited cytotoxicity against the HT-29 cell line with ED50 values of 4.9, 1.7, 1.7, 2.3, and 9.1 microM, respectively. In an ELISA NF-kappaB assay, compounds 5-7, 9, and 10 inhibited p65 activation with IC50 values of 15.9, 12.1, 3.2, 11.3, and 19.0 microM, respectively, and 6 showed p50 inhibitory activity with an IC50 value of 7.5 microM. Alpha-mangostin (5) was further tested in an in vivo hollow fiber assay, using HT-29, LNCaP, and MCF-7 cells, but it was found to be inactive at the highest dose tested (20 mg/kg).
Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen).[Pubmed:16536578]
J Agric Food Chem. 2006 Mar 22;54(6):2077-82.
As part of ongoing research on cancer chemopreventive agents from botanical dietary supplements, Garcinia mangostana L. (commonly known as mangosteen) was selected for detailed study. Repeated chromatography of a CH2Cl2-soluble extract of the pericarp led to the isolation of two new highly oxygenated prenylated xanthones, 8-hydroxycudraxanthone G (1) and mangostingone [7-methoxy-2-(3-methyl-2-butenyl)-8-(3-methyl-2-oxo-3-butenyl)-1,3,6-trihydroxyxa nthone, 2], together with 12 known xanthones, cudraxanthone G (3), 8-deoxygartanin (4), garcimangosone B (5), garcinone D (6), garcinone E (7), gartanin (8), 1-Isomangostin (9), alpha-mangostin (10), gamma-mangostin (11), mangostinone (12), smeathxanthone A (13), and tovophyllin A (14). The structures of compounds 1 and 2 were elucidated by spectroscopic data analysis. Except for compound 2, which was isolated as a minor component, the antioxidant activities of all isolates were determined using authentic and morpholinosydnonimine-derived peroxynitrite methods, and compounds 1, 8, 10, 11, and 13 were the most active. Alpha-mangostin (10) inhibited 7,12-dimethylbenz[alpha]anthracene-induced preneoplastic lesions in a mouse mammary organ culture assay with an IC50 of 1.0 microg/mL (2.44 microM).
Pharmacological profile of mangostin and its derivatives.[Pubmed:314790]
Arch Int Pharmacodyn Ther. 1979 Jun;239(2):257-69.
Mangostin (M), a naturally occurring xanthone in the rinds of the fruits of Garcinia mangostana Linn. (Guttiferae) and its derivatives such as 3-0-methyl mangostin (MM), 3,6-di-O-methyl mangostin (DM), 1-Isomangostin (IM), mangostin triacetate (MT), mangostin 3,6-di-O-(tetra acetyl) glucoside (MTG) and mangostin-6,6-di-O-glucoside (MOG) were screened for various pharmacological effects in experimental animals. With the exception of DM all the test compounds produced CNS depression characterised by ptosis, sedation, decreased motor activity, potentiation of pentobarbital sleeping time and ether anaesthesia in mice and rats. None of the compounds exhibited analgesic, antipyretic and anticonvulsant effects. With the exception of MOG, none of the test compounds produced significant effects on the cardiovascular system of frogs and dogs. MOG produced myocardial stimulation and a rise in blood pressure which was partially blocked by propranolol. M, IM and MT produced pronounced antiinflammatory activity both by intraperitoneal and oral routes in rats as tested by carrageenininduced hind paw oedema, cotton pellet implantation and granuloma pouch techniques. Antiinflammatory activity for M, IM and MT was observed even in bilaterally adrenalectomised rats. M, IM and MT did not produce any mast cell membrane stabilising effect and the degranulation effect of polymyxin B, diazoxide and Triton X-100 on rat peritoneal mast cells in vitro was not prevented. M, IM and MT did not alter the prothrombin time of albino rats. M alone produced significant antiulcer activity in rats.


