Xanthoangelol BCAS# 132998-81-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 132998-81-3 | SDF | Download SDF |
| PubChem ID | 10409180 | Appearance | Powder |
| Formula | C25H28O5 | M.Wt | 408.49 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
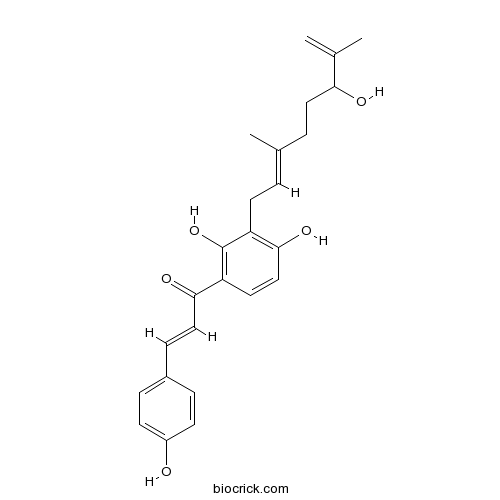
| Chemical Name | (E)-1-[2,4-dihydroxy-3-[(2E)-6-hydroxy-3,7-dimethylocta-2,7-dienyl]phenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one | ||
| SMILES | CC(=C)C(CCC(=CCC1=C(C=CC(=C1O)C(=O)C=CC2=CC=C(C=C2)O)O)C)O | ||
| Standard InChIKey | NCHZAFAGBAEJJJ-BAYITLGHSA-N | ||
| Standard InChI | InChI=1S/C25H28O5/c1-16(2)22(27)13-5-17(3)4-11-20-24(29)15-12-21(25(20)30)23(28)14-8-18-6-9-19(26)10-7-18/h4,6-10,12,14-15,22,26-27,29-30H,1,5,11,13H2,2-3H3/b14-8+,17-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Xanthoangelol B and its derivative PM-56 were shown to bind directly to SaeS and inhibit its histidine kinase activity, which suggests a possibility of a broad spectrum inhibitor of histidine kinases. Xanthoangelol B inhibited the phenylephrine-induced vasoconstriction most strongly, and the inhibitory mechanism of it on phenylephrine-induced vasoconstriction might involve the direct inhibition of smooth muscle functions through the reduction of [Ca2+]i elevation without affecting EDRF/NO production. |
| Targets | Calcium Channel | NO | NMDA receptor |
| In vitro | Artery relaxation by chalcones isolated from the roots of Angelica keiskei.[Pubmed: 11345693 ]Planta Med. 2001 Apr;67(3):230-5.
|
| Kinase Assay | Total Synthesis of Xanthoangelol B and Its Various Fragments: Toward Inhibition of Virulence Factor Production of Staphylococcus aureus.[Pubmed: 30388007 ]J Med Chem. 2018 Dec 13;61(23):10473-10487.As an alternative strategy to fight antibiotic resistance, two-component systems (TCSs) have emerged as novel targets. Among TCSs, master virulence regulators that control the expression of multiple virulence factors are considered as excellent antivirulence targets. In Staphylococcus aureus, virulence factor expression is tightly regulated by a few master regulators, including the SaeRS TCS.
|

Xanthoangelol B Dilution Calculator

Xanthoangelol B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.448 mL | 12.2402 mL | 24.4804 mL | 48.9608 mL | 61.201 mL |
| 5 mM | 0.4896 mL | 2.448 mL | 4.8961 mL | 9.7922 mL | 12.2402 mL |
| 10 mM | 0.2448 mL | 1.224 mL | 2.448 mL | 4.8961 mL | 6.1201 mL |
| 50 mM | 0.049 mL | 0.2448 mL | 0.4896 mL | 0.9792 mL | 1.224 mL |
| 100 mM | 0.0245 mL | 0.1224 mL | 0.2448 mL | 0.4896 mL | 0.612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isololiolide
Catalog No.:BCN8703
CAS No.:38274-00-9
- Bryonamide B
Catalog No.:BCN8702
CAS No.:5942-25-6
- Cinchonine hydrochloride
Catalog No.:BCN8701
CAS No.:5949-11-1
- Tangshenoside I
Catalog No.:BCN8700
CAS No.:117278-74-7
- Trillikamtoside R
Catalog No.:BCN8699
CAS No.:2098642-71-6
- Secologanoside
Catalog No.:BCN8698
CAS No.:59472-23-0
- Picropodophyllol
Catalog No.:BCN8697
CAS No.:3811-15-2
- 5,7,2',4'-Tetrahydroxy-8,3'-di(gamma,gamma-dimethylallyl)-isoflavanone
Catalog No.:BCN8696
CAS No.:141846-47-1
- Clitorin
Catalog No.:BCN8695
CAS No.:55804-74-5
- 3-O-Acetyl-11-hydroxy-beta-boswellic acid
Catalog No.:BCN8694
CAS No.:146019-25-2
- Isorhamnetin 3-robinobioside
Catalog No.:BCN8693
CAS No.:53584-69-3
- Notoginsenoside FP2
Catalog No.:BCN8692
CAS No.:1004988-75-3
- 5,6,7,4'-tetrahydroxyisoflavone-6,7-di-O-beta-D-glucopyranoside
Catalog No.:BCN8705
CAS No.:1219001-04-3
- Trikamsteroside E
Catalog No.:BCN8706
CAS No.:952579-37-2
- Pilosidine
Catalog No.:BCN8707
CAS No.:229971-57-7
- Isocarlinoside
Catalog No.:BCN8708
CAS No.:83151-90-0
- Asiaticoside B
Catalog No.:BCN8709
CAS No.:125265-68-1
- Quercetin 3-O-beta-(6''-p-coumaroyl)glucopyranosyl(1->2)-alpha-L-rhamnopyranoside
Catalog No.:BCN8711
CAS No.:143061-65-8
- Methyl caffeate acid
Catalog No.:BCN8712
CAS No.:3843-74-1
- Bryonamide A
Catalog No.:BCN8713
CAS No.:75268-14-3
- Glycinol
Catalog No.:BCN8714
CAS No.:69393-95-9
- 2-O-Acetyl-20-hydroxyecdysone
Catalog No.:BCN8715
CAS No.:19536-25-5
- Indole-3-acetic acid
Catalog No.:BCN8716
CAS No.:87-51-4
- 1-Isomangostin
Catalog No.:BCN8717
CAS No.:19275-44-6
Total Synthesis of Xanthoangelol B and Its Various Fragments: Toward Inhibition of Virulence Factor Production of Staphylococcus aureus.[Pubmed:30388007]
J Med Chem. 2018 Nov 14.
As an alternative strategy to fight antibiotic resistance, two-component systems (TCSs) have emerged as novel targets. Among TCSs, master virulence regulators that control the expression of multiple virulence factors are considered as excellent antivirulence targets. In Staphylococcus aureus, virulence factor expression is tightly regulated by a few master regulators, including the SaeRS TCS. In this study, we used a SaeRS GFP-reporter system to screen natural compound inhibitors of SaeRS, and identified Xanthoangelol B 1, a prenylated chalcone from Angelica keiskei as a hit. We have synthesized 1 and its derivative PM-56 and shown that 1 and PM-56 both had excellent inhibitory potency against the SaeRS TCS, as demonstrated by various in vitro and in vivo experiments. As a mode of action, 1 and PM-56 were shown to bind directly to SaeS and inhibit its histidine kinase activity, which suggests a possibility of a broad spectrum inhibitor of histidine kinases.


