SecologanosideCAS# 59472-23-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 59472-23-0 | SDF | Download SDF |
| PubChem ID | 14136854 | Appearance | White powder |
| Formula | C16H22O11 | M.Wt | 390.34 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in methan | ||
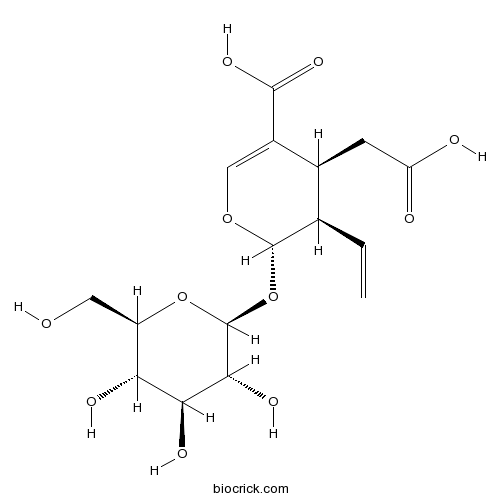
| Chemical Name | (2~{S},3~{R},4~{S})-4-(carboxymethyl)-3-ethenyl-2-[(2~{S},3~{R},4~{S},5~{S},6~{R})-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3,4-dihydro-2~{H}-pyran-5-carboxylic acid | ||
| SMILES | C=CC1C(C(=COC1OC2C(C(C(C(O2)CO)O)O)O)C(=O)O)CC(=O)O | ||
| Standard InChIKey | RGTONEMDTVVDMY-GRTPNEQMSA-N | ||
| Standard InChI | InChI=1S/C16H22O11/c1-2-6-7(3-10(18)19)8(14(23)24)5-25-15(6)27-16-13(22)12(21)11(20)9(4-17)26-16/h2,5-7,9,11-13,15-17,20-22H,1,3-4H2,(H,18,19)(H,23,24)/t6-,7+,9-,11-,12+,13-,15+,16+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Secologanoside shows anti-complementary, allelopathic, and anti-elastase activities. |
| In vitro | Biological activities of triterpenoids from Poraqueiba sericea stems.[Pubmed: 27736194 ]Nat Prod Res. 2017 Jun;31(11):1333-1338.
Anti-complementary phenolic acids from Lonicera japonica.[Pubmed: 26080557]Zhongguo Zhong Yao Za Zhi. 2015 Jan;40(2):269-74.To study the anti-complementary phenolic acids from Lonicera japonica.
|
| Structure Identification | Planta Med. 1996 Aug;62(4):365-8.Secoiridoids and xanthones from Gentianella nitida.[Pubmed: 17252473]
Phytochemistry (Oxford), 2000, 55(2):131-140.Allelochemicals of the tropical weed Sphenoclea zeylanica.[Reference: WebLink]Nine plant growth inhibitors were isolated from the tropical weed Sphenoclea zeylanica, which shows allelopathic properties.
|

Secologanoside Dilution Calculator

Secologanoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5619 mL | 12.8093 mL | 25.6187 mL | 51.2374 mL | 64.0467 mL |
| 5 mM | 0.5124 mL | 2.5619 mL | 5.1237 mL | 10.2475 mL | 12.8093 mL |
| 10 mM | 0.2562 mL | 1.2809 mL | 2.5619 mL | 5.1237 mL | 6.4047 mL |
| 50 mM | 0.0512 mL | 0.2562 mL | 0.5124 mL | 1.0247 mL | 1.2809 mL |
| 100 mM | 0.0256 mL | 0.1281 mL | 0.2562 mL | 0.5124 mL | 0.6405 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Picropodophyllol
Catalog No.:BCN8697
CAS No.:3811-15-2
- 5,7,2',4'-Tetrahydroxy-8,3'-di(gamma,gamma-dimethylallyl)-isoflavanone
Catalog No.:BCN8696
CAS No.:141846-47-1
- Clitorin
Catalog No.:BCN8695
CAS No.:55804-74-5
- 3-O-Acetyl-11-hydroxy-beta-boswellic acid
Catalog No.:BCN8694
CAS No.:146019-25-2
- Isorhamnetin 3-robinobioside
Catalog No.:BCN8693
CAS No.:53584-69-3
- Notoginsenoside FP2
Catalog No.:BCN8692
CAS No.:1004988-75-3
- Beta-Hydroxyisovalerylshikonin
Catalog No.:BCN8691
CAS No.:7415-78-3
- Clematichinenoside AR
Catalog No.:BCN8690
CAS No.:761425-93-8
- Conicasterol
Catalog No.:BCN8689
CAS No.:76758-18-4
- Licochalcone E
Catalog No.:BCN8688
CAS No.:864232-34-8
- Licochalcone D
Catalog No.:BCN8687
CAS No.:144506-15-0
- Isopropyl ferulate
Catalog No.:BCN8686
CAS No.:59831-94-6
- Trillikamtoside R
Catalog No.:BCN8699
CAS No.:2098642-71-6
- Tangshenoside I
Catalog No.:BCN8700
CAS No.:117278-74-7
- Cinchonine hydrochloride
Catalog No.:BCN8701
CAS No.:5949-11-1
- Bryonamide B
Catalog No.:BCN8702
CAS No.:5942-25-6
- Isololiolide
Catalog No.:BCN8703
CAS No.:38274-00-9
- Xanthoangelol B
Catalog No.:BCN8704
CAS No.:132998-81-3
- 5,6,7,4'-tetrahydroxyisoflavone-6,7-di-O-beta-D-glucopyranoside
Catalog No.:BCN8705
CAS No.:1219001-04-3
- Trikamsteroside E
Catalog No.:BCN8706
CAS No.:952579-37-2
- Pilosidine
Catalog No.:BCN8707
CAS No.:229971-57-7
- Isocarlinoside
Catalog No.:BCN8708
CAS No.:83151-90-0
- Asiaticoside B
Catalog No.:BCN8709
CAS No.:125265-68-1
- Quercetin 3-O-beta-(6''-p-coumaroyl)glucopyranosyl(1->2)-alpha-L-rhamnopyranoside
Catalog No.:BCN8711
CAS No.:143061-65-8
Secoiridoids and xanthones from Gentianella nitida.[Pubmed:17252473]
Planta Med. 1996 Aug;62(4):365-8.
From Gentianella nitida twelve known metabolites were isolated and identified by HPLC-UV and/or by spectroscopic methods as Secologanoside, amaroswerin, amarogentin (secoiridoids), isoorientin (C-glucosylflavone), mangiferin, demethylbellidifolin 8-O-glucoside, norswertianine 1-O-glucoside, swertianine 1-O-primeveroside, swertianine 8-O-glucoside, norswertianine, demethylbellidifolin, and swertianine (xanthone glycosides and aglycones). Secologanoside is reported here for the first time in Gentianaceae species; the antioxidant mangiferin was obtained as the major compound in good yield.


