Licochalcone DCAS# 144506-15-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 144506-15-0 | SDF | Download SDF |
| PubChem ID | 10473311 | Appearance | Powder |
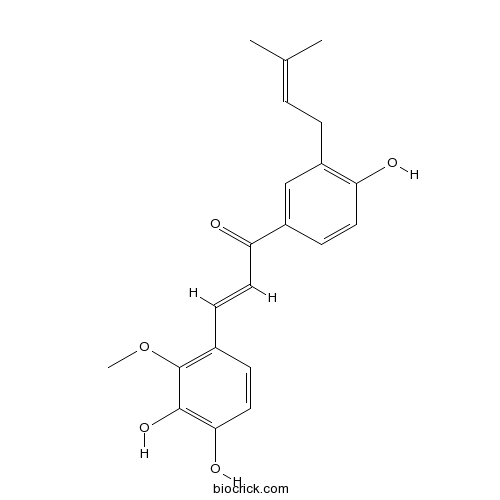
| Formula | C21H22O5 | M.Wt | 354.40 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | DMSO : 125 mg/mL (352.71 mM; Need ultrasonic) | ||
| Chemical Name | (E)-3-(3,4-dihydroxy-2-methoxyphenyl)-1-[4-hydroxy-3-(3-methylbut-2-enyl)phenyl]prop-2-en-1-one | ||
| SMILES | CC(=CCC1=C(C=CC(=C1)C(=O)C=CC2=C(C(=C(C=C2)O)O)OC)O)C | ||
| Standard InChIKey | RETRVWFVEFCGOK-RMKNXTFCSA-N | ||
| Standard InChI | InChI=1S/C21H22O5/c1-13(2)4-5-15-12-16(8-10-18(15)23)17(22)9-6-14-7-11-19(24)20(25)21(14)26-3/h4,6-12,23-25H,5H2,1-3H3/b9-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Licochalcone D has anti-inflammatory, and anti-allergic activities, it shows suppression ability of nitric oxide (NO) production, it also suppresses degranulation by decreasing the intracellular Ca2+ level and tyrosine phosphorylation of ERK in RBL-2H3 cells. Licochalcone D has cardioprotective potential against myocardial ischemia/reperfusion injury in langendorff-perfused rat hearts. It may be a potential drug for human melanoma treatment by inhibiting proliferation, inducing apoptosis via the mitochondrial pathway and blocking cell migration and invasion. |
| Targets | ROS | MMP(e.g.TIMP) | Bcl-2/Bax | Caspase | Akt | p65 | NF-kB | p38MAPK | PARP | NOS | NO | MEK | ERK | Calcium Channel | PKA |
| In vitro | Licochalcone D induces apoptosis and inhibits migration and invasion in human melanoma A375 cells.[Pubmed: 29565458]Oncol Rep. 2018 May;39(5):2160-2170.The aim of the present study was to determine the effects of Licochalcone D (LD) on the apoptosis and migration and invasion in human melanoma A375 cells. Synthesis of licochalcone analogues with increased anti-inflammatory activity.[Pubmed: 24316124 ]Bioorg Med Chem Lett. 2014 Jan 1;24(1):181-5.Licohalcones have been reported to have various biological activities. However, most of licochalcones also showed cytotoxicity even though their versitile utilities.
|
| In vivo | Cardioprotective Effect of Licochalcone D against Myocardial Ischemia/Reperfusion Injury in Langendorff-Perfused Rat Hearts.[Pubmed: 26058040]PLoS One. 2015 Jun 9;10(6):e0128375.Flavonoids are important components of 'functional foods', with beneficial effects on cardiovascular function. |
| Kinase Assay | Glycyrrhiza inflata-derived chalcones, Licochalcone A, Licochalcone B and Licochalcone D, inhibit phosphorylation of NF-kappaB p65 in LPS signaling pathway.[Pubmed: 19291859]Licochalcones suppress degranulation by decreasing the intracellular Ca2+ level and tyrosine phosphorylation of ERK in RBL-2H3 cells.[Pubmed: 20399908 ]Int Immunopharmacol. 2010 Jul;10(7):769-76.Mast cells play a key role in allergic inflammation by releasing various mediators, such as histamine, serotonin, leukotrienes and cytokines. A signaling cascade of events activated by stimulation with antigens contributes to the regulation of mast cell degranulation. While various anti-inflammatory and anti-allergic drugs have been developed that inhibit degranulation of mast cells, the inhibitory mechanism has been poorly understood. Licochalcone A (Lico A) is a retrochalcone isolated from the root of Xinjiang liquorice and has been reported to exhibit various biological activities such as anti-inflammatory activity. Int Immunopharmacol. 2009 Apr;9(4):499-507.Licorice root has been used as a traditional medicine for the treatment of gastric ulcer, bronchial asthma and inflammation. Licochalcone A is a major component of Xinjiang licorice, Glycyrrhiza inflata. Previously we showed that Licochalcone A significantly inhibited LPS-induced NF-kappaB transcriptional activation by abrogating the phosphorylation of NF-kappaB p65 at serine 276. |

Licochalcone D Dilution Calculator

Licochalcone D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8217 mL | 14.1084 mL | 28.2167 mL | 56.4334 mL | 70.5418 mL |
| 5 mM | 0.5643 mL | 2.8217 mL | 5.6433 mL | 11.2867 mL | 14.1084 mL |
| 10 mM | 0.2822 mL | 1.4108 mL | 2.8217 mL | 5.6433 mL | 7.0542 mL |
| 50 mM | 0.0564 mL | 0.2822 mL | 0.5643 mL | 1.1287 mL | 1.4108 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2822 mL | 0.5643 mL | 0.7054 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isopropyl ferulate
Catalog No.:BCN8686
CAS No.:59831-94-6
- Isolindleyin
Catalog No.:BCN8685
CAS No.:87075-18-1
- Glabrol
Catalog No.:BCN8684
CAS No.:59870-65-4
- Protohypericin
Catalog No.:BCN8683
CAS No.:548-03-8
- Huzhangoside B
Catalog No.:BCN8682
CAS No.:94795-70-7
- Cafestol
Catalog No.:BCN8681
CAS No.:469-83-0
- Perisesaccharide C
Catalog No.:BCN8680
CAS No.:1311473-28-5
- Kudinoside D
Catalog No.:BCN8679
CAS No.:173792-61-5
- Flemiphilippinin A
Catalog No.:BCN8678
CAS No.:140366-64-9
- Magnaldehyde B
Catalog No.:BCN8677
CAS No.:92829-72-6
- Lappaol C
Catalog No.:BCN8676
CAS No.:64855-00-1
- 13-Methylberberine
Catalog No.:BCN8675
CAS No.:54260-72-9
- Licochalcone E
Catalog No.:BCN8688
CAS No.:864232-34-8
- Conicasterol
Catalog No.:BCN8689
CAS No.:76758-18-4
- Clematichinenoside AR
Catalog No.:BCN8690
CAS No.:761425-93-8
- Beta-Hydroxyisovalerylshikonin
Catalog No.:BCN8691
CAS No.:7415-78-3
- Notoginsenoside FP2
Catalog No.:BCN8692
CAS No.:1004988-75-3
- Isorhamnetin 3-robinobioside
Catalog No.:BCN8693
CAS No.:53584-69-3
- 3-O-Acetyl-11-hydroxy-beta-boswellic acid
Catalog No.:BCN8694
CAS No.:146019-25-2
- Clitorin
Catalog No.:BCN8695
CAS No.:55804-74-5
- 5,7,2',4'-Tetrahydroxy-8,3'-di(gamma,gamma-dimethylallyl)-isoflavanone
Catalog No.:BCN8696
CAS No.:141846-47-1
- Picropodophyllol
Catalog No.:BCN8697
CAS No.:3811-15-2
- Secologanoside
Catalog No.:BCN8698
CAS No.:59472-23-0
- Trillikamtoside R
Catalog No.:BCN8699
CAS No.:2098642-71-6
Cardioprotective Effect of Licochalcone D against Myocardial Ischemia/Reperfusion Injury in Langendorff-Perfused Rat Hearts.[Pubmed:26058040]
PLoS One. 2015 Jun 9;10(6):e0128375.
Flavonoids are important components of 'functional foods', with beneficial effects on cardiovascular function. The present study was designed to investigate whether Licochalcone D (LD) could be a cardioprotective agent in ischemia/reperfusion (I/R) injury and to shed light on its possible mechanism. Compared with the I/R group, LD treatment enhanced myocardial function (increased LVDP, dp/dtmax, dp/dtmin, HR and CR) and suppressed cardiac injury (decreased LDH, CK and myocardial infarct size). Moreover, LD treatment reversed the I/R-induced cleavage of caspase-3 and PARP, resulting in a significant decrease in proinflammatory factors and an increase in antioxidant capacity in I/R myocardial tissue. The mechanisms underlying the antiapoptosis, antiinflammation and antioxidant effects were related to the activation of the AKT pathway and to the blockage of the NF-kappaB/p65 and p38 MAPK pathways in the I/R-injured heart. Additionally, LD treatment markedly activated endothelial nitric oxide synthase (eNOS) and reduced nitric oxide (NO) production. The findings indicated that LD had real cardioprotective potential and provided support for the use of LD in myocardial I/R injury.
Glycyrrhiza inflata-derived chalcones, Licochalcone A, Licochalcone B and Licochalcone D, inhibit phosphorylation of NF-kappaB p65 in LPS signaling pathway.[Pubmed:19291859]
Int Immunopharmacol. 2009 Apr;9(4):499-507.
Licorice root has been used as a traditional medicine for the treatment of gastric ulcer, bronchial asthma and inflammation. Licochalcone A is a major component of Xinjiang licorice, Glycyrrhiza inflata. Previously we showed that Licochalcone A significantly inhibited LPS-induced NF-kappaB transcriptional activation by abrogating the phosphorylation of NF-kappaB p65 at serine 276. Glycyrrhiza inflata contains not only Licochalcone A but also Licochalcone B, Licochalcone C, Licochalcone D, Echinatin and Isoliquiritigenin, harboring the common structure of chalcones. No chalcones had any effect on LPS-induced IkappaB degradation, nuclear translocation and DNA binding activity of NF-kappaB p65; however, we observed that Licochalcone B and Licochalcone D significantly inhibited LPS-induced phosphorylation at serine 276 and transcriptional activation of NF-kappaB, the same as Licochalcone A. Interestingly, we also found that Licochalcone A, Licochalcone B and Licochalcone D effectively inhibited LPS-induced activation of PKA, which is required for the phosphorylation of NF-kappaB p65 at serine 276. Consequently, Licochalcone B and Licochalcone D significantly reduced the LPS-induced production of NO, TNFalpha and MCP-1. On the other hand, Licochalcone C, Echinatin and Isoliquitigenin failed to inhibit LPS-induced NF-kappaB activation. These findings suggest that the anti-inflammatory effect of Glycyrrhiza inflata is ascribable to the potent inhibition of NF-kappaB by Licochalcone A, Licochalcone B and Licochalcone D.
Licochalcones suppress degranulation by decreasing the intracellular Ca2+ level and tyrosine phosphorylation of ERK in RBL-2H3 cells.[Pubmed:20399908]
Int Immunopharmacol. 2010 Jul;10(7):769-76.
Mast cells play a key role in allergic inflammation by releasing various mediators, such as histamine, serotonin, leukotrienes and cytokines. A signaling cascade of events activated by stimulation with antigens contributes to the regulation of mast cell degranulation. While various anti-inflammatory and anti-allergic drugs have been developed that inhibit degranulation of mast cells, the inhibitory mechanism has been poorly understood. Licochalcone A (Lico A) is a retrochalcone isolated from the root of Xinjiang liquorice and has been reported to exhibit various biological activities such as anti-inflammatory activity. We examined the effects of Lico A and related chalcones on degranulation in a rat basophilic leukemia cell line, RBL-2H3. Whereas Lico A and licochalcone C (Lico C) exhibited inhibitory activity with cytotoxicity, Licochalcone D (Lico D) significantly inhibited the degranulation in RBL-2H3 cells with low cytotoxicity. Moreover, Lico D significantly inhibited the Ca2+ influx and phosphorylation of extracellular signal regulated kinase (ERK) and MEK. These results suggest that Lico D inhibits mast cell degranulation via the inhibition of both extracellular Ca2+ influx and activation of the MEK-ERK pathway.
Synthesis of licochalcone analogues with increased anti-inflammatory activity.[Pubmed:24316124]
Bioorg Med Chem Lett. 2014 Jan 1;24(1):181-5.
Licohalcones have been reported to have various biological activities. However, most of licochalcones also showed cytotoxicity even though their versitile utilities. Licochalcones B and D, which have common substituents at aromatic ring B, are targeted to modify the structure at aromatic ring A for inflammatory studies. Licochalcone Derivatives (1-6) thus prepared are compared for their suppression ability of nitric oxide (NO) production and showed 9.94, 4.72, 10.1, 4.85, 2.37 and 4.95muM of IC50 values, respectively.
Licochalcone D induces apoptosis and inhibits migration and invasion in human melanoma A375 cells.[Pubmed:29565458]
Oncol Rep. 2018 May;39(5):2160-2170.
The aim of the present study was to determine the effects of Licochalcone D (LD) on the apoptosis and migration and invasion in human melanoma A375 cells. Cell proliferation was determined by sulforhodamine B assay. Apoptosis was assessed by Hoechst 33258 and Annexin VFITC/PI staining and JC1 assay. Total intracellular reactive oxygen species (ROS) was examined by DCFHDA. Wound healing and Transwell assays were used to detect migration and invasion of the cells. The activities of matrix metalloproteinase (MMP2 and MMP9) were assessed via gelatin zymography. Tumor growth in vivo was evaluated in C57BL/6 mice. RTPCR, qPCR, ELISA and western blot analysis were utilized to measure the mRNA and protein levels. Our results showed that LD inhibited the proliferation of A375 and SKMEL5 cells in a concentrationdependent manner. After treatment with LD, A375 cells displayed obvious apoptotic characteristics, and the number of apoptotic cells was significantly increased. Proapoptotic protein Bax, caspase9 and caspase3 were upregulated, while antiapoptotic protein Bcl2 was downregulated in the LDtreated cells. Meanwhile, LD induced the loss of mitochondrial membrane potential (DeltaPsim) and increased the level of ROS. ROS production was inhibited by the cotreatment of LD and free radical scavenger Nacetylcysteine (NAC). Furthermore, LD also blocked A375 cell migration and invasion in vitro which was associated with the downregulation of MMP9 and MMP2. Finally, intragastric administration of LD suppressed tumor growth in the mouse xenograft model of murine melanoma B16F0 cells. These results suggest that LD may be a potential drug for human melanoma treatment by inhibiting proliferation, inducing apoptosis via the mitochondrial pathway and blocking cell migration and invasion.


