Licochalcone ECAS# 864232-34-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

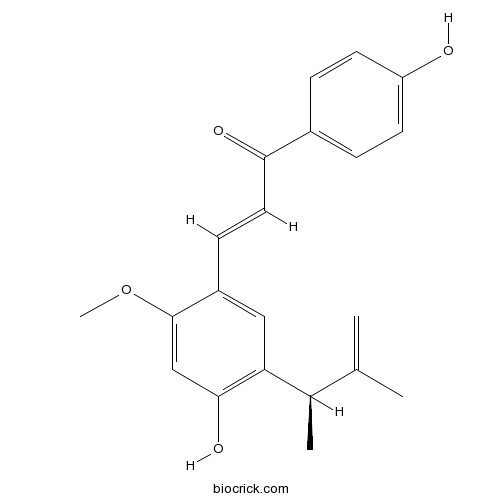
| Cas No. | 864232-34-8 | SDF | Download SDF |
| PubChem ID | 134715164 | Appearance | Powder |
| Formula | C21H22O4 | M.Wt | 338.40 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | DMSO : 125 mg/mL (369.39 mM; Need ultrasonic) | ||
| Chemical Name | (E)-3-[4-hydroxy-2-methoxy-5-[(2R)-3-methylbut-3-en-2-yl]phenyl]-1-(4-hydroxyphenyl)prop-2-en-1-one | ||
| SMILES | CC(C1=C(C=C(C(=C1)C=CC(=O)C2=CC=C(C=C2)O)OC)O)C(=C)C | ||
| Standard InChIKey | SWPKMTGYQGHLJS-DNGMOHDESA-N | ||
| Standard InChI | InChI=1S/C21H22O4/c1-13(2)14(3)18-11-16(21(25-4)12-20(18)24)7-10-19(23)15-5-8-17(22)9-6-15/h5-12,14,22,24H,1H2,2-4H3/b10-7+/t14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Licochalcone E is a potential LXRβ agonist. 2. Licochalcone E may be used for the treatment of hepatotoxicity, and primarily exhibits its protective role through a PPARγ/NF-κB-mediated pathway. 3. Licochalcone E exhibits potential chemopreventive effects and warrants further developed as a chemotherapeutic agent against oral cancer. 4. Licochalcone E exhibits potent anti-inflammatory property in 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse ear edema and lipopolysaccharide (LPS)-stimulated RAW 264.7 murine macrophage models. 5. Licochalcone E suppresses lung metastasis in the 4T1 mammary orthotopic cancer model. 6. Licochalcone E shows potent antimicrobial property against Staphylococcus aureus. 7. Licochalcone E is a potential activator of the Nrf2/ARE-dependent pathway and is therapeutically relevant not only to oxidative-stress-related neurodegeneration but also inflammatory responses of microglial cells both in vitro and in vivo. 8. Licochalcone E has an antidiabetic effect, it increases the levels of PPARγ expression, at least in part, via the stimulation of Akt signals and functions as a PPARγ partial agonist. 9. Licochalcone E reduces chronic allergic contact dermatitis and inhibits IL-12p40 production through down-regulation of NF-kappa B. 10. Licochalcone E shows cytotoxicity against the human tumor cell lines. |
| Targets | Liver X Receptor | PPAR | TNF-α | NF-kB | NOS | COX | JNK | ERK | PGE | p38MAPK | IkB | AP-1 | p65 | VEGFR | Bcl-2/Bax | Caspase | MMP(e.g.TIMP) | Nrf2 | IKK |

Licochalcone E Dilution Calculator

Licochalcone E Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9551 mL | 14.7754 mL | 29.5508 mL | 59.1017 mL | 73.8771 mL |
| 5 mM | 0.591 mL | 2.9551 mL | 5.9102 mL | 11.8203 mL | 14.7754 mL |
| 10 mM | 0.2955 mL | 1.4775 mL | 2.9551 mL | 5.9102 mL | 7.3877 mL |
| 50 mM | 0.0591 mL | 0.2955 mL | 0.591 mL | 1.182 mL | 1.4775 mL |
| 100 mM | 0.0296 mL | 0.1478 mL | 0.2955 mL | 0.591 mL | 0.7388 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Licochalcone D
Catalog No.:BCN8687
CAS No.:144506-15-0
- Isopropyl ferulate
Catalog No.:BCN8686
CAS No.:59831-94-6
- Isolindleyin
Catalog No.:BCN8685
CAS No.:87075-18-1
- Glabrol
Catalog No.:BCN8684
CAS No.:59870-65-4
- Protohypericin
Catalog No.:BCN8683
CAS No.:548-03-8
- Huzhangoside B
Catalog No.:BCN8682
CAS No.:94795-70-7
- Cafestol
Catalog No.:BCN8681
CAS No.:469-83-0
- Perisesaccharide C
Catalog No.:BCN8680
CAS No.:1311473-28-5
- Kudinoside D
Catalog No.:BCN8679
CAS No.:173792-61-5
- Flemiphilippinin A
Catalog No.:BCN8678
CAS No.:140366-64-9
- Magnaldehyde B
Catalog No.:BCN8677
CAS No.:92829-72-6
- Lappaol C
Catalog No.:BCN8676
CAS No.:64855-00-1
- Conicasterol
Catalog No.:BCN8689
CAS No.:76758-18-4
- Clematichinenoside AR
Catalog No.:BCN8690
CAS No.:761425-93-8
- Beta-Hydroxyisovalerylshikonin
Catalog No.:BCN8691
CAS No.:7415-78-3
- Notoginsenoside FP2
Catalog No.:BCN8692
CAS No.:1004988-75-3
- Isorhamnetin 3-robinobioside
Catalog No.:BCN8693
CAS No.:53584-69-3
- 3-O-Acetyl-11-hydroxy-beta-boswellic acid
Catalog No.:BCN8694
CAS No.:146019-25-2
- Clitorin
Catalog No.:BCN8695
CAS No.:55804-74-5
- 5,7,2',4'-Tetrahydroxy-8,3'-di(gamma,gamma-dimethylallyl)-isoflavanone
Catalog No.:BCN8696
CAS No.:141846-47-1
- Picropodophyllol
Catalog No.:BCN8697
CAS No.:3811-15-2
- Secologanoside
Catalog No.:BCN8698
CAS No.:59472-23-0
- Trillikamtoside R
Catalog No.:BCN8699
CAS No.:2098642-71-6
- Tangshenoside I
Catalog No.:BCN8700
CAS No.:117278-74-7
Licochalcone E present in licorice suppresses lung metastasis in the 4T1 mammary orthotopic cancer model.[Pubmed:23625311]
Cancer Prev Res (Phila). 2013 Jun;6(6):603-13.
We investigated whether Licochalcone E (LicE), a phenolic constituent of licorice, inhibits mammary tumor growth and metastasis using animal and cell culture models. 4T1 mammary carcinoma cells were injected into the mammary fat pads of syngeneic BALB/c mice. Starting 7 days after the injection, the mice received LicE (7 or 14 mg/kg body weight/day) via oral gavage for 25 days. LicE suppressed solid tumor growth and lung metastasis, but did not exhibit kidney or liver toxicity. In tumor tissues, LicE treatment induced a reduction in the expression of Ki67, cyclins, and cyclin-dependent kinases and stimulated apoptosis with increased expression of Bax and cleaved caspase-3 but decreased expression of Bcl-2. In addition, LicE decreased expression of CD31, vascular endothelial growth factor (VEGF)-A and C, VEGF-receptor 2, lymphatic vessel endothelial receptor-1, CD45, cyclooxygenase-2, inducible nitric oxide synthase, and hypoxia inducible factor-1alpha in tumor tissues. In lung tissues, LicE reduced the levels of proinflammatory cytokines and angiogenesis/metastasis-related proteins. In mammary cancer cell cultures, LicE (5-20 mumol/L) dose dependently inhibited cell migration and invasion. LicE inhibited secretion of matrix metalloproteinase-9, urokinase-type plasminogen activator and VEGF-A, and stimulated secretion of tissue inhibitor of metalloproteinase-2 in MDA-MB-231 cells. In addition, LicE inhibited tube formation of vascular endothelial cells. We show that LicE administration suppressed tumor growth and lung metastasis in the mouse model in conjunction with LicE inhibition of cell migration, invasion, and tube formation in vitro. Reduced tumor growth and metastasis in LicE-treated mice may be, at least in part, attributed to reduced inflammation and tumor angiogenesis.
Licochalcone E reduces chronic allergic contact dermatitis and inhibits IL-12p40 production through down-regulation of NF-kappa B.[Pubmed:20601178]
Int Immunopharmacol. 2010 Sep;10(9):1119-26.
Licochalcone, a constituent of licorice, has antitumor, antimicrobial, and anti-inflammatory effects. Recently, Licochalcone E was isolated from the roots of Glycyrrhiza inflata and its biological functions are not fully examined. In this study, we investigated its ability to modulate production of IL-12p40, a common subunit of IL-12 and IL-23. Licochalcone E dose-dependently inhibited IL-12p40 production from lipopolysaccharide-stimulated RAW264.7 macrophage cells. The repressive effect was mapped to a region in the IL-12 gene promoter containing a binding site for NF-kappaB. Furthermore, Licochalcone E decreased binding to the NF-kappaB site in RAW264.7 macrophage cells. Using a chronic allergic contact dermatitis model induced by repeated application of oxazolone, we showed that Licochalcone E inhibited the increased IL-12p40 expression and ear thickness induced by oxazolone. Taken together, Licochalcone E inhibits IL-12p40 production and has therapeutic potential to reduce skin inflammation.
Mechanisms by which licochalcone e exhibits potent anti-inflammatory properties: studies with phorbol ester-treated mouse skin and lipopolysaccharide-stimulated murine macrophages.[Pubmed:23708096]
Int J Mol Sci. 2013 May 24;14(6):10926-43.
In this study we found that Licochalcone E (LicE), a recently isolated retrochalcone from Glycyrrhiza inflata, exhibits potent anti-inflammatory effects in 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse ear edema and lipopolysaccharide (LPS)-stimulated RAW 264.7 murine macrophage models. Topical application of LicE (0.5-2 mg) effectively inhibited TPA-induced (1) ear edema formation; (2) phosphorylation of stress-activated protein kinase/c-Jun-N-terminal kinase (SAPK/JNK), c-Jun, and extracellular signal regulated kinase 1/2; and (3) expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 proteins in mouse skin. The treatment of RAW 264.7 cells with LicE (2.5-7.5 mumol/L) induced a profound reduction in LPS-induced (1) release of NO and prostaglandin E2; (2) mRNA expression and secretion of interleukin (IL)-6, IL-1beta and tumor necrosis factor-alpha; (3) promoter activity of iNOS and COX-2 and expression of their corresponding mRNAs and proteins; (4) activation of AKT, p38 mitogen activated protein kinase (MAPK), SAPK/JNK and c-Jun; (5) phosphorylation of inhibitor of kappaB (IkappaB) kinase-alphabeta and IkappaBalpha, degradation of IkappaBalpha, translocation of p65 (RelA) to the nucleus and transcriptional activity of nuclear factor (NF)-kappaB; and (6) transcriptional activity of activator protein (AP)-1. These results indicate that the LicE inhibition of NF-kappaB and AP-1 transcriptional activity through the inhibition of AKT and MAPK activation contributes to decreases in the expression of pro-inflammatory cytokines and the inducible enzymes iNOS and COX-2.
Licochalcone E has an antidiabetic effect.[Pubmed:21840191]
J Nutr Biochem. 2012 Jul;23(7):759-67.
Licochalcone E (lico E) is a retrochalcone isolated from the root of Glycyrrhiza inflata. Retrochalcone compounds evidence a variety of pharmacological profiles, including anticancer, antiparasitic, antibacterial, antioxidative and superoxide-scavenging properties. In this study, we evaluated the biological effects of lico E on adipocyte differentiation in vitro and obesity-related diabetes in vivo. We employed 3T3-L1 preadipocyte and C3H10T1/2 stem cells for in vitro adipocyte differentiation study and diet-induced diabetic mice for in vivo study. The presence of lico E during adipogenesis induced adipocyte differentiation to a significant degree, particularly at the early induction stage. Licochalcone E evidenced weak, but significant, peroxisome proliferator-activated receptor gamma (PPARgamma) ligand-binding activity. Two weeks of lico E treatment lowered blood glucose levels and serum triglyceride levels in the diabetic mice. Additionally, treatment with lico E resulted in marked reductions in adipocyte size and increases in the mRNA expression levels of PPARgamma in white adipose tissue (WAT). Licochalcone E was also shown to significantly stimulate Akt signaling in epididymal WAT. In conclusion, lico E increases the levels of PPARgamma expression, at least in part, via the stimulation of Akt signals and functions as a PPARgamma partial agonist, and this increased PPARgamma expression enhances adipocyte differentiation and increases the population of small adipocytes, resulting in improvements in hyperglycemia and hyperlipidemia under diabetic conditions.
Antimicrobial activity of licochalcone E against Staphylococcus aureus and its impact on the production of staphylococcal alpha-toxin.[Pubmed:22573157]
J Microbiol Biotechnol. 2012 Jun;22(6):800-5.
Licochalcone E was firstly isolated from licorice root in 2005, which belongs to the retrochalcone family. Studies on the biological activities of Licochalcone E were in the initial stage. In the study, we demonstrated that Licochalcone E has potent antimicrobial property against Staphylococcus aureus. Furthermore, via hemolysis, Western blot, and real-time RT-PCR assays, we have shown that subinhibitory concentrations of Licochalcone E dosedependently reduces the production of alpha-toxin in both methicillin-sensitive S. aureus (MSSA) and methicillinresistant S. aureus (MRSA). The data suggest that Licochalcone E may deserve further investigation as a potential therapeutic against S. aureus infections, or the structure of Licochalcone E may be used as a basis for chemical synthesis of novel anti-S. aureus compounds.
Topoisomerase I inhibition and cytotoxicity of licochalcones A and E from Glycyrrhiza inflata.[Pubmed:17424936]
Arch Pharm Res. 2007 Mar;30(3):313-6.
Licochalcones A (1) and E (2), retrochalcones or reversely constructed chalcones, isolated from the roots of Glycyrrhiza inflata, were evaluated for their cytotoxicities against four different human tumor cell lines; A549 (lung), SK-OV-3 (ovarian), SK-MEL-2 (melanoma) and HCT-15 (colon), using the sulforhodamine B (SRB) assay. The effects of these compounds toward the DNA topoisomerase I (topo I) inhibitory activity were also measured using the supercoiled DNA unwinding assay. All compounds showed moderate cytotoxicities against the four different human tumor cell lines and inhibited the topo I activity in dose-dependent manners. The inhibition of topo I by licochalcones A (1) and E (2) may explain the cytotoxicities of these compounds against the human tumor cell lines.
[Discovery of potential LXRbeta agonists from Chinese herbs using molecular simulation methods].[Pubmed:28920350]
Zhongguo Zhong Yao Za Zhi. 2016 Aug;41(16):3065-3071.
Liver X receptor beta (LXRbeta) has been a new target in the treatment of hyperlipemia, which was related to the cholesterol homeostasis. In this study, the quantitative pharmacophores were constructed by 3D-QSAR pharmacophore (Hypogen) method based on the LXRbeta agonists. The optimal pharmacophore model containing one hydrogen bond acceptor, two hydrophobics and one ring aromatic was obtained based on five assessment indictors, including the correlation between predicted value and experimental value of the compounds in training set (correlation), Deltacost of the models (Deltacost), hit rate of active compounds (HRA), identification of effectiveness index (IEI) and comprehensive evaluation index (CAI). And the values of the five assessment indicators were 0.95, 128.65, 84.44%, 2.58 and 2.18 respectively. The best model as a query to screen the traditional Chinese medicine database (TCMD), a list of 309 compounds was obtained andwere then refined using Libdock program. Finally, based on the screening rules of the Libdock score of initial compound and the key interactions between initial compound and receptor, four compounds, demethoxycurcumin, isolicoflavonol, Licochalcone E and silydianin, were selected as potential LXRbeta agonists. The molecular simulation methods were high-efficiency and time-saving to obtainthe potential LXRbeta agonists, which could provide assistance for further researchingnovel anti-hyperlipidemia drugs.
Licochalcone E activates Nrf2/antioxidant response element signaling pathway in both neuronal and microglial cells: therapeutic relevance to neurodegenerative disease.[Pubmed:22227268]
J Nutr Biochem. 2012 Oct;23(10):1314-23.
Oxidative stress and neuroinflammation are hallmarks of neurodegenerative diseases, which do not play independently but work synergistically through complex interactions exacerbating neurodegeneration. Therefore, the mechanism that is directly implicated in controlling oxidative stress and inflammatory response could be an attractive strategy to prevent the onset and/or delay the progression of neurodegenerative diseases. The transcription factor nuclear factor-E2-related factor-2 (Nrf2) is the guardian of redox homeostasis by regulating a battery of antioxidant and phase II detoxification genes, which are relevant to defense mechanism against oxidative stress and inflammatory responses. In this study, we show that a recently identified Glycyrrhiza-inflata-derived chalcone, Licochalcone E (Lico-E), attenuates lipopolysaccharide-induced inflammatory responses in microglial BV2 cells and protects dopaminergic SH-SY5Y cells from 6-hydroxydopamine cytotoxicity. Lico-E activates Nrf2-antioxidant response element (ARE) system and up-regulates downstream NAD(P)H:quinone oxidoreductase 1 (NQO1) and heme oxygenase-1 (HO-1). Anti-inflammatory and cytoprotective effects of Lico-E are attenuated in siRNA-mediated Nrf2-silencing cells as well as in the presence with specific inhibitor of HO-1 or NQO1, respectively. Lico-E also has neuroprotective effect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced nigrostriatal dopaminergic neurodegeneration in mice, with up-regulation of HO-1 and NQO1 in the substantia nigra of the brain. This study demonstrates that Lico-E is a potential activator of the Nrf2/ARE-dependent pathway and is therapeutically relevant not only to oxidative-stress-related neurodegeneration but also inflammatory responses of microglial cells both in vitro and in vivo.
Licochalcone E protects against carbon tetrachlorideinduced liver toxicity by activating peroxisome proliferator-activated receptor gamma.[Pubmed:28849019]
Mol Med Rep. 2017 Oct;16(4):5269-5276.
The present study aimed to investigate the hepatoprotective role of Licochalcone E (LCE) and its mechanism of action in a mouse model of carbon tetrachloride (CCl4)induced liver toxicity. Hepatotoxicity was induced in Kunming mice via an intraperitoneal injection (IP) of CCl4, 10 ml/kg body weight, diluted with corn oil at a 1:500 ratio. LCE was administered once a day for 7 days (IP) as pretreatment at a dose of 5 mg/kg/day. The levels of Creactive protein (CRP) and tumor necrosis factor (TNF)alpha were analyzed to determine the inflammation status. The levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were analyzed using ELISA assays. Liver ultrastructure was observed via optical microscopy. The mRNA and protein expression levels of peroxisome proliferatoractivated receptor (PPAR)gamma, and nuclear factor (NF)kappaB were assayed using quantitative polymerase chain reaction and western blot analysis, respectively. Pretreatment with LCE decreased levels of ALT, AST, CRP and TNFalpha, and NFkappaB expression in the experimental hepatotoxicity mice model induced by CCl4. In addition, LCE increased the expression of PPARgamma and normalized the hepatic histoarchitecture. However, the effects of LCE were reversed by cotreatment with the PPARgamma inhibitor GW9662. The present study suggests that LCE may be used for the treatment of hepatotoxicity, and primarily exhibits its protective role through a PPARgamma/NFkappaBmediated pathway.


