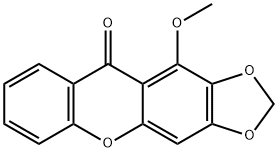
1-Methoxy-2,3-methylenedioxyxanthoneCAS# 63625-05-8 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 63625-05-8 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C15H10O5 | M.Wt | 270.25 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Synonyms | 11-Methoxy-10H-1,3-dioxolo[4,5-b]xanthen-10-one | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

1-Methoxy-2,3-methylenedioxyxanthone Dilution Calculator

1-Methoxy-2,3-methylenedioxyxanthone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7003 mL | 18.5014 mL | 37.0028 mL | 74.0056 mL | 92.5069 mL |
| 5 mM | 0.7401 mL | 3.7003 mL | 7.4006 mL | 14.8011 mL | 18.5014 mL |
| 10 mM | 0.37 mL | 1.8501 mL | 3.7003 mL | 7.4006 mL | 9.2507 mL |
| 50 mM | 0.074 mL | 0.37 mL | 0.7401 mL | 1.4801 mL | 1.8501 mL |
| 100 mM | 0.037 mL | 0.185 mL | 0.37 mL | 0.7401 mL | 0.9251 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1,2,3-Trimethoxyxanthone
Catalog No.:BCX1999
CAS No.:27460-10-2
- 3-Hydroxy-1,2-dimethoxyxanthone
Catalog No.:BCX1998
CAS No.:20362-27-0
- 5,6-Dihydroxy-8-methoxyflavone-7-O-glucuronide
Catalog No.:BCX1997
CAS No.:1169879-99-5
- (6S,9R)-2-Hydroxy-4-(2,6,6-trimethyl-4-oxo-cyclohex-2-enyl)-butyric acid
Catalog No.:BCX1996
CAS No.:2089289-59-6
- 5,6,7,8-Tetrahydroxy-3',4'-dimethoxyflavone
Catalog No.:BCX1995
CAS No.:2930091-30-6
- Anhydroperiplogenone
Catalog No.:BCX1994
CAS No.:1247-04-7
- Digitoxigenin glucomethyloside
Catalog No.:BCX1993
CAS No.:40950-57-0
- Strospeside
Catalog No.:BCX1992
CAS No.:595-21-1
- Quercetin-3-O-α-(6'''-caffeoylglucosyl-β-1,2-rhamnoside)
Catalog No.:BCX1991
CAS No.:851222-75-8
- Digitoxigenin monodigitoxoside
Catalog No.:BCX1990
CAS No.:18404-43-8
- Isolithospermic acid
Catalog No.:BCX1899
CAS No.:1217879-76-9
- Ipalbinium
Catalog No.:BCX1898
CAS No.:110200-24-3
- 1,3-Dihydroxy-2-methoxyxanthone
Catalog No.:BCX2001
CAS No.:87339-74-0
- Digitalin
Catalog No.:BCX2002
CAS No.:752-61-4
- T-Muurolol
Catalog No.:BCX2003
CAS No.:19912-62-0
- T-Cadinol
Catalog No.:BCX2004
CAS No.:5937-11-1
- Paeonicluside
Catalog No.:BCX2005
CAS No.:448231-30-9
- Schiarisanrin D
Catalog No.:BCX2006
CAS No.:188943-88-6
- (-)-cis-Calamenene
Catalog No.:BCX2007
CAS No.:483-77-2
- (-)-Torreyol
Catalog No.:BCX2008
CAS No.:19435-97-3
- 17β-Tenacigenin B
Catalog No.:BCX2009
CAS No.:863767-79-7
- 2,5-Dihydroxy-7-methoxyflavanone
Catalog No.:BCX2010
CAS No.:35486-66-9
- 5,8-Dihydroxy-6,7,3′,4′-tetramethoxyflavone
Catalog No.:BCX2011
CAS No.:683278-67-3
- Ananonin A
Catalog No.:BCX2012
CAS No.:1314021-67-4
Normalization of the H3K9me2/H3K14ac-DeltaFosB pathway in the nucleus accumbens underlying the reversal of morphine-induced behavioural and synaptic plasticity by Compound 511.[Pubmed:36252464]
Phytomedicine. 2023 Jan;108:154467.
BACKGROUND: Although opioid agonist-based treatments are considered the first-line treatment for opioid use disorders, nonopioid alternatives are urgently needed to combat the inevitable high relapse rates. Compound 511 is a formula derived from ancient traditional Chinese medical literature on opiate rehabilitation. Previously, we observed that Compound 511 could effectively prevent the acquisition of conditioned place preference (CPP) during early morphine exposure. However, its effects on drug-induced reinstatement remain unclear. PURPOSE: This study aims to estimate the potential of Compound 511 for the therapeutic intervention of opioid relapse in rodent models and explore the potential mechanisms underlying the observed actions. STUDY DESIGN/METHODS: The CPP and locomotor sensitization paradigm were established to evaluate the therapeutic effect of Compound 511 treatment on morphine-induced neuroadaptations, followed by immunofluorescence and western blot (WB) analysis of the synaptic markers PSD-95 and Syn-1. Furthermore, several addiction-associated transcription factors and epigenetic marks were examined by qPCR and WB, respectively. Furthermore, the key active ingredients and targets of Compound 511 were further excavated by network pharmacology approach and experimental validation. RESULTS: The results proved that Compound 511 treatment during abstinence blunted both the reinstatement of morphine-evoked CPP and locomotor sensitization, accompanied by the normalization of morphine-induced postsynaptic plasticity in the nucleus accumbens (NAc). Additionally, Compound 511 was shown to exert a selectively repressive influence on morphine-induced hyperacetylation at H3K14 and a reduction in H3K9 dimethylation as well as DeltaFosB activation and accumulation in the NAc. Finally, two herbal ingredients of Compound 511 and six putative targets involved in the regulation of histone modification were identified. CONCLUSION: Our findings indicated that Compound 511 could block CPP reinstatement and locomotor sensitization predominantly via the reversal of morphine-induced postsynaptic plasticity through epigenetic mechanisms. Additionally, 1-Methoxy-2,3-methylenedioxyxanthone and 1,7-dimethoxyxanthone may serve as key ingredients of Compound 511 by targeting specific epigenetic enzymes. This study provided an efficient nonopioid treatment against opioid addiction.
[Chemical constituents of Polygala tenuifolia root].[Pubmed:25857159]
Zhong Yao Cai. 2014 Sep;37(9):1594-6.
OBJECTIVE: To study the chemical constituents from the root of Polygala tenuifolia. METHODS: Silica gel,Sephadex LH-20 and ODS chromatographic techniques were used to study the chemical constituents of Polygala tenuifolia, and the chemical structures were elucidated by application of spectral data. RESULTS: Eight-compounds were obtained and their structure were identified as 7-hydroxy-1-Methoxy-2,3-methylenedioxyxanthone(1), 1, 7-dihydroxy-2, 3-dimethoxyxanth one(2), 1,3,6-trihydroxy-2, 7-dimethoxyxanthone(3),7-hydroxy-1,2, 3-trimethoxyxanthone(4), 1,2,3,6, 7-pentamethoxyxanthone(5), 1,3, 7-trihydroxy-2, 6-dimethanoxyxanthone (6),7-hydroxy-1-methoxyxanthone(7) and 1,7-dihydroxy-3,4-dimethoxyxanthone(8). CONCLUSION: Compounds 1,6,7 and 8 are isolated from Polygala tenuifolia for the first time.
[Chemical constituents in roots of Polygala fallax and their anti-oxidation activities in vitro].[Pubmed:16110862]
Zhongguo Zhong Yao Za Zhi. 2005 Jun;30(11):827-30.
OBJECTIVE: To study the chemical constituents in roots of P. fallax and their anti-oxidation activities in vitro. METHOD: Column chromatographic techniques were employed for isolation and purification of chemical constituents of the plant. The structures were elucidated on the basis of the spectral evidence and the physical and chemical character. The isolated compounds were screened with four anti-oxidation models in vitro. RESULT: Seven xanthones, 1,7-dihydroxy-2,3-methylenedioxyxanthone (1), 1-Methoxy-2,3-methylenedioxyxanthone (2), 3-hydroxy-1,2-dimethoxyxanthone (3), 1,6,7-trihydroxy-2,3-dimethoxyxanthone (4), 7-hydroxy-1-Methoxy-2,3-methylenedioxyxanthone (5), 1,3-dihydroxy-2-methoxyxanthone (6) and 1,3,7-trihydroxy-2-methoxyxanthone (7), were isolated from the roots of P. fallax. And compounds 1 - 7 showed different anti-oxidation activities in the different pharmacological models. CONCLUSION: Compounds 2, 3, 5 and 7 were isolated from this plant for the first time. Xanthones from this plant showed anti-oxidation activities. The pharmacological activities of the pure compounds from this plant were also reported for the first time.
Xanthones from the roots of Polygala caudata and their antioxidation and vasodilatation activities in vitro.[Pubmed:15856419]
Planta Med. 2005 Apr;71(4):372-5.
Three new xanthones, 2-hydroxy-1,6,7-trimethoxyxanthone (1), 1,4-dimethoxy-2,3-methylenedioxyxanthone (2), and 7-hydroxy-1,2-dimethoxyxanthone (3), together with five known compounds, 2,7-dihydroxy-1-methoxyxanthone (4), 1-Methoxy-2,3-methylenedioxyxanthone (5), 7-hydroxy-1-methoxyxanthone (6), euxanthone (1,7-dihydroxyxanthone) (7), and gentitein (1,3,7-trihydroxyxanthone) (8), were isolated from the roots of Polygala caudata. Their structures were established on the basis of spectral evidence. In the antioxidation activity screening in vitro with luminol chemiluminescence methods, compounds 1 - 5 and 7 and 8 showed H2O2 scavenger activity, with a scavenging effect of 58.4 - 94.5% at 10 microg/mL, and 26.0 - 84.7% at 2 microg/mL. Compounds 4 and 8 also exhibited scavenging effects on the reactive oxygen free radicals produced by macrophage respiratory bursts, with a scavenging effect of 71.7% and 63.4% at 10 microg/mL, 41.2% and 47.8% at 2 microg/mL, respectively. In the vasodilatation assay, compounds 4 - 7 exhibited relaxing activity on the contractions evoked by KCl in Wistar rat thoracic aorta rings in a dose-dependent manner.
[Studies on chemical constituents of Polygala arillata buch-ham].[Pubmed:11498872]
Yao Xue Xue Bao. 1997 May;32(5):360-2.
Two new compounds were isolated from the roots of Polygala arillata Buch-Ham. On the basis of chemical reactions and spectral (UV, IR, MS, 1HNMR, DIFNOE, 13CNMR) analysis, they were identified as 1,3-dihydroxy-2-methoxyxanthone(I) and 7-hydroxy-1-Methoxy-2,3-methylenedioxyxanthone(II). Pharmacological study indicated that I and II have inhibitory effect on aldose reductase activity.


