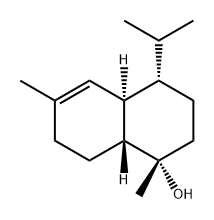
T-CadinolCAS# 5937-11-1 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 5937-11-1 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C15H26O | M.Wt | 206.41 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Synonyms | 10βH-Cadin-4-en-10-ol,Cedrelanol,10-Epicadinol | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

T-Cadinol Dilution Calculator

T-Cadinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8447 mL | 24.2236 mL | 48.4473 mL | 96.8945 mL | 121.1182 mL |
| 5 mM | 0.9689 mL | 4.8447 mL | 9.6895 mL | 19.3789 mL | 24.2236 mL |
| 10 mM | 0.4845 mL | 2.4224 mL | 4.8447 mL | 9.6895 mL | 12.1118 mL |
| 50 mM | 0.0969 mL | 0.4845 mL | 0.9689 mL | 1.9379 mL | 2.4224 mL |
| 100 mM | 0.0484 mL | 0.2422 mL | 0.4845 mL | 0.9689 mL | 1.2112 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- T-Muurolol
Catalog No.:BCX2003
CAS No.:19912-62-0
- Digitalin
Catalog No.:BCX2002
CAS No.:752-61-4
- 1,3-Dihydroxy-2-methoxyxanthone
Catalog No.:BCX2001
CAS No.:87339-74-0
- 1-Methoxy-2,3-methylenedioxyxanthone
Catalog No.:BCX2000
CAS No.:63625-05-8
- 1,2,3-Trimethoxyxanthone
Catalog No.:BCX1999
CAS No.:27460-10-2
- 3-Hydroxy-1,2-dimethoxyxanthone
Catalog No.:BCX1998
CAS No.:20362-27-0
- 5,6-Dihydroxy-8-methoxyflavone-7-O-glucuronide
Catalog No.:BCX1997
CAS No.:1169879-99-5
- (6S,9R)-2-Hydroxy-4-(2,6,6-trimethyl-4-oxo-cyclohex-2-enyl)-butyric acid
Catalog No.:BCX1996
CAS No.:2089289-59-6
- 5,6,7,8-Tetrahydroxy-3',4'-dimethoxyflavone
Catalog No.:BCX1995
CAS No.:2930091-30-6
- Anhydroperiplogenone
Catalog No.:BCX1994
CAS No.:1247-04-7
- Digitoxigenin glucomethyloside
Catalog No.:BCX1993
CAS No.:40950-57-0
- Strospeside
Catalog No.:BCX1992
CAS No.:595-21-1
- Paeonicluside
Catalog No.:BCX2005
CAS No.:448231-30-9
- Schiarisanrin D
Catalog No.:BCX2006
CAS No.:188943-88-6
- (-)-cis-Calamenene
Catalog No.:BCX2007
CAS No.:483-77-2
- (-)-Torreyol
Catalog No.:BCX2008
CAS No.:19435-97-3
- 17β-Tenacigenin B
Catalog No.:BCX2009
CAS No.:863767-79-7
- 2,5-Dihydroxy-7-methoxyflavanone
Catalog No.:BCX2010
CAS No.:35486-66-9
- 5,8-Dihydroxy-6,7,3′,4′-tetramethoxyflavone
Catalog No.:BCX2011
CAS No.:683278-67-3
- Ananonin A
Catalog No.:BCX2012
CAS No.:1314021-67-4
- Benzoyloxokadsuranol
Catalog No.:BCX2013
CAS No.:130252-47-0
- Heteroclitin F
Catalog No.:BCX2015
CAS No.:144049-67-2
- Tenuifoliose B
Catalog No.:BCX2016
CAS No.:139682-02-3
- Tenuifoliose J
Catalog No.:BCX2017
CAS No.:147742-14-1
Promising Antileishmanial Activity of Micromeria nervosa Essential Oil: In Vitro and In Silico Studies.[Pubmed:38675696]
Molecules. 2024 Apr 19;29(8):1876.
The present study aimed to evaluate the leishmanicidal potential of the essential oil (EO) of Micromeria (M.) nervosa and to investigate its molecular mechanism of action by qPCR. Furthermore, in silicointeraction study of the major M. nervosa EO compounds with the enzyme cytochrome P450 sterol 14alpha-demethylase (CYP51) was also performed. M. nervosa EO was analyzed by gas chromatography-mass spectrometry (GC-MS). Results showed that alpha-pinene (26.44%), T-Cadinol (26.27%), caryophyllene Oxide (7.73 +/- 1.04%), and alpha-Cadinene (3.79 +/- 0.12%) are the major compounds of M. nervosa EO. However, limited antioxidant activity was observed, as this EO was ineffective in neutralizing DPPH free radicals and in inhibiting beta-carotene bleaching. Interestingly, it displayed effective leishmanicidal potential against promastigote (IC(50) of 6.79 and 5.25 mug/mL) and amastigote (IC(50) of 8.04 and 7.32 mug/mL) forms of leishmania (L.) infantum and L. major, respectively. Molecular mechanism investigation showed that M. nervosa EO displayed potent inhibition on the thiol regulatory pathway. Furthermore, a docking study of the main components of the EO with cytochrome P450 sterol 14alpha-demethylase (CYP51) enzyme revealed that T-Cadinol exhibited the best binding energy values (-7.5 kcal/mol), followed by alpha-cadinene (-7.3 kcal/mol) and caryophyllene oxide (-7 kcal/mol). These values were notably higher than that of the conventional drug fluconazole showing weaker binding energy (-6.9 kcal/mol). These results suggest that M. nervosa EO could serve as a potent and promising candidate for the development of alternative antileishmanial agent in the treatment of leishmaniasis.
First investigation of the chemical composition, antioxidant, antimicrobial and larvicidal activities of the essential oil of the subspecies Ononis angustissima Lam. subsp. filifolia Murb.[Pubmed:38247329]
Nat Prod Res. 2024 Jan 22:1-16.
This study is the first to explore the essential oil of Ononis angustissima Lam. subsp. filifolia Murb., a subspecies growing in the Algerian northeastern Sahara. The chemical composition was evaluated by GC/GC-EIMS. Antioxidant activity was evaluated using two methods. Thirty-four (91.6%) individual components were identified. The main constituents were linalool (12.6%), hexahydrofarnesylacetone (8.4%), beta-eudesmol (6.6%), alpha-cadinol (6.4%) and T-Cadinol (6.1%). The findings provide a chemical basis for understanding relationships between North African subspecies, supporting botanical and genetic classification. The oil exhibited moderate scavenging activity against DPPH radicals (IC(50 )= 102.30 microg/ml) and high activity in the beta-carotene bleaching assay (91.346%). Antimicrobial tests revealed effectiveness against Gram-positive bacteria (Staphylococcus aureus ATCC 25923 and ATCC 43300), limited impact on Gram-negative bacteria (Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922), and good inhibition against Aspergillus niger and Scedosporium apiospermum. A notable larvicidal activity was observed against Date Moth, particularly on L2 larvae.
Evaluation of the Capparis Herbacea Willd's Chemistry, Antioxidant and Cytotoxic Activity.[Pubmed:38018181]
Antiinflamm Antiallergy Agents Med Chem. 2023;22(4):261-272.
BACKGROUND: The Capparidaceae family includes the medicinal herb Capparis herbacea Willd. The aerial and underground parts of plant C.herbacea were studied for their chemical composition, antioxidant, and cytotoxic properties. METHODS: Using gas chromatography with mass spectrometric detection (7890A/5975C), 94 chemicals were identified in ethanol extract from leaves, roots, seeds, and stems of C. herbacea. Main components were (leaves) phytol 18.16%, hexanedioic acid, bis(2-ethylhexyl) ester 16.75%, vitamin E 11.95%, (roots) sucrose 13.94%, hexadecanoic acid, ethylester 22.80%, octadecanoic acid, ethylester 37.77%; (seeds) hexadecanoic acid, ethylester 13.96%, ethyl9.cis.,11.trans.-octadecadienoate 48.54%, bis(2-ethylhexyl) phthalate 9.77%; (stems) 1-propene-1,2,3-tricarboxylic acid, tributyl ester 42.69%, and tributylacetylcitrate 19.63%. Nine components were identified in the makeup of the C. herbacea sample's essential oil using the method of chromatography-mass spectrometry. RESULTS: The main components were (in%): T-Cadinol (29.56), meta-cymene (16.12), pulegone (14.11), and sigma-amorphene (12.26). Chloroform and methanol extracts of Capparis herbacia roots at concentrations of 1 mg/ml showed higher average antioxidant activity, while ethyl acetate root extract at concentrations of 0.75 and 1 mg/ml showed higher average antioxidant activity compared to gallic acid AOA. CONCLUSION: In addition, plant extracts have cytotoxic activity. Essential oils of leaves and stems, fruit and roots of Capparis herbacia plants exhibited cytotoxicity, all larvae died, and larval mortality was 96%.
Activity of essential oils from leaves, flower buds and stems of Tetradenia riparia on Rhipicephalus (Boophilus) microplus larvae.[Pubmed:36820731]
Rev Bras Parasitol Vet. 2023 Feb 20;32(1):e013522.
Around the world, the main problems of livestock are caused by ectoparasites, however, commercial acaracide are toxic to the environment and detrimental to One Health. Therefore, research has increasingly focused on development of natural products as alternatives for tick control. The purpose of this study was to evaluate the larvicidal effect on Rhipicephalus (Boophilus) microplus, through use of essential oils (EOs) extracted from the leaves, flower buds and stems of Tetradenia riparia. The chemical composition of these EOs was determined through gas chromatography coupled to mass spectrometry (GC-MS). They were tested on larvae at concentrations of 100.000 to 40 microg/mL, using the larval packet test and under semi-natural conditions. The main class of compounds in the chemical composition was sesquiterpenes (both oxygenates and hydrocarbons), whereas the predominant compounds in the leaves, flower buds and stems were 14-hydroxy-9-epi-caryophyllene, T-Cadinol and 6-7-dehydroroyleanone, respectively. The leaves proved to be the most effective, with highest larvicidal activity (LC99.9 = 83.53 microg/mL). When tested under semi-natural conditions, the oils obtained efficiency above 98% in all compound tests. The results indicated that these EOs were effective against R. (B.) microplus larvae in vitro and ex-situ, proving that this plant has bioactive molecules with significant larvicidal activity.
Neutrophil Immunomodulatory Activity of Nerolidol, a Major Component of Essential Oils from Populus balsamifera Buds and Propolis.[Pubmed:36501438]
Plants (Basel). 2022 Dec 6;11(23):3399.
Propolis is a resinous mixture of substances collected and processed from various botanical sources by honeybees. Black poplar (Populus balsamifera L.) buds are one of the primary sources of propolis. Despite their reported therapeutic properties, little is known about the innate immunomodulatory activity of essential oils from P. balsamifera and propolis. In the present studies, essential oils were isolated from the buds of P. balsamifera and propolis collected in Montana. The main components of the essential oil from P. balsamifera were E-nerolidol (64.0%), 1,8-cineole (10.8%), benzyl benzoate (3.7%), alpha-terpinyl acetate (2.7%), alpha-pinene (1.8%), o-methyl anisol (1.8%), salicylaldehyde (1.8%), and benzyl salicylate (1.6%). Likewise, the essential oil from propolis was enriched with E-nerolidol (14.4%), cabreuva oxide-VI (7.9%), alpha-bisabolol (7.1%), benzyl benzoate (6.1%), beta-eudesmol (3.6%), T-Cadinol (3.1%), 2-methyl-3-buten-2-ol (3.1%), alpha-eudesmol (3.0%), fokienol (2.2%), nerolidol oxide derivative (1.9%), decanal (1.8%), 3-butenyl benzene (1.5%), 1,4-dihydronaphthalene (1.5%), selina-4,11-diene (1.5%), alpha-cadinol (1.5%), linalool (1.4%), gamma-cadinene (1.4%), 2-phenylethyl-2-methyl butyrate (1.4%), 2-methyl-2-butenol (1.3%), octanal (1.1%), benzylacetone (1.1%), and eremoligenol (1.1%). A comparison between P. balsamifera and propolis essential oils demonstrated that 22 compounds were found in both essential oil samples. Both were enriched in E-nerolidol and its derivatives, including cabreuva oxide VI and nerolidol oxides. P. balsamifera and propolis essential oils and pure nerolidol activated Ca(2+) influx in human neutrophils. Since these treatments activated neutrophils, the essential oil samples were also evaluated for their ability to down-regulate the neutrophil responses to subsequent agonist activation. Indeed, treatment with P. balsamifera and propolis essential oils inhibited subsequent activation of these cells by the N-formyl peptide receptor 1 (FPR1) agonist fMLF and the FPR2 agonist WKYMVM. Likewise, nerolidol inhibited human neutrophil activation induced by fMLF (IC(50) = 4.0 muM) and WKYMVM (IC(50) = 3.7 muM). Pretreatment with the essential oils and nerolidol also inhibited human neutrophil chemotaxis induced by fMLF, again suggesting that these treatments down-regulated human neutrophil responses to inflammatory chemoattractants. Finally, reverse pharmacophore mapping predicted several potential kinase targets for nerolidol. Thus, our studies have identified nerolidol as a potential anti-inflammatory modulator of human neutrophils.
Corrigendum: (-)-T-Cadinol, a Sesquiterpene Isolated From Casearia sylvestris (Salicaceae)-Displayed In Vitro Activity and Causes Hyperpolarization of the Membrane Potential of Trypanosoma cruzi.[Pubmed:35370638]
Front Pharmacol. 2022 Mar 14;13:865432.
[This corrects the article DOI: 10.3389/fphar.2021.734127.].
Teucrium polium (L.): Phytochemical Screening and Biological Activities at Different Phenological Stages.[Pubmed:35268662]
Molecules. 2022 Feb 25;27(5):1561.
The aim of the present study was to investigate the changes in the content of phytochemical compounds and in vitro antioxidant, antibacterial, and anti-inflammatory activities of Teucrium polium L. aerial parts and root methanolic extracts at different phenological stages (vegetative, flowering, and seeding). The T. polium extracts were analyzed using gas chromatography-mass spectrometry (GC-MS), and their antioxidant properties were tested with the 2,2-diphenyl-1-picrylhydrazyl (DPPH), nitric oxide (NO), ferrous ions (Fe2+), and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) methods. Forty-nine compounds were identified with the majority of germacrene D, T-Cadinol, beta-pinene, carvacrol, bicyclogermacrene, alpha-pinene, and limonene. The results show that the extracts significantly differ between different phenological stages of the plant material used in terms of the phytochemical composition (total phenolic compounds, total flavonoids, total alkaloids, and total saponin contents) and bioactivities (antioxidant, antibacterial, and anti-inflammatory) (p < 0.05). The highest total contents of phenolics (72.4 +/- 2.5 mg gallic acid equivalent (GAE)/g dry weight), flavonoids (36.2 +/- 3.1 mg quercetin equivalent (QE)/g dry weight), alkaloids (105.7 +/- 2.8 mg atropine equivalent (AE)/g dry weight), and saponins (653 +/- 6.2 mg escin equivalent (EE)/g dry weight), as well as antioxidant, antibacterial, and anti-inflammatory activities, were measured for the extract of the aerial parts obtained at the flowering stage. The minimum inhibitory concentration (MIC) values for the extracts were varied within 9.4-300 microg/mL, while the minimum bactericidal concentration (MBC) values were varied within 18.75-600 microg/mL. In addition, they were more active on Gram-positive bacteria than Gram-negative bacteria. The data of this work confirm that the T. polium extracts have significant biological activity and hence can be used in the pharmaceutical industry, clinical applications, and medical research, as well as cosmetic and food industries.
(-)-T-Cadinol-a Sesquiterpene Isolated From Casearia sylvestris (Salicaceae)-Displayed In Vitro Activity and Causes Hyperpolarization of the Membrane Potential of Trypanosoma cruzi.[Pubmed:34803682]
Front Pharmacol. 2021 Nov 3;12:734127.
Chagas disease is caused by the protozoan parasite Trypanosoma cruzi and affects 6-8 million people worldwide, mainly from developing countries. The treatment is limited to two approved nitro-derivatives, nifurtimox and benznidazole, with several side effects and reduced efficacy. Casearia sylvestris has been used in folk medicine as an antiseptic and cicatrizing in skin diseases. In the present work, the hexane phase from the MeOH extract from the leaves of Casearia sylvestris afforded a fraction composed by the sesquiterpene T-Cadinol, which was chemically characterized by NMR and HRMS. The activity of T-Cadinol was evaluated against T. cruzi, and IC(50) values of 18 (trypomastigotes) and 15 (amastigotes) microM were established. The relation between the mammalian toxicity and the antiparasitic activity resulted in a selectivity index >12. Based on this promising activity, the mechanism of action was investigated by different approaches using fluorescent-based techniques such as plasma membrane permeability, plasma membrane electric potential, mitochondrial membrane electric potential, reactive oxygen species, and the intracellular calcium (Ca(2+)) levels. The obtained results demonstrated that T-Cadinol affected neither the parasite plasma membrane nor the electric potential of the membrane. Nevertheless, this compound induced a mitochondrial impairment, resulting in a hyperpolarization of the membrane potential, with decreased levels of reactive oxygen species. No alterations in Ca(2+) levels were observed, suggesting that T-Cadinol may affect the single mitochondria of the parasite. This is the first report about the occurrence of T-Cadinol in C. sylvestris, and our data suggest this sesquiterpene as an interesting hit compound for future optimizations in drug discovery studies for Chagas disease.
Phytochemical composition and pharmacological activities of Teucrium polium L. collected from eastern Turkey.[Pubmed:38143885]
Turk J Chem. 2021 Nov 22;46(1):269-282.
Teucrium species that belong to the family Lamiaceae have been traditionally used for their medicinal properties. T. polium is one the most widespread members of the genus for its use in the treatment of several diseases. In this study, the essential oil and phenolic composition of the aerial parts from T. polium were assessed by GC-FID, GC/MS, and LC-MS/MS as well as for its total phenolic content. Several extracts such as n-hexane, chloroform, methanol, and infusion were prepared and their antimicrobial, antioxidant, and also acetylcholinesterase activities were studied. According to GC/MS results, beta -caryophyllene (8.8%), T-Cadinol (6.2%), (E)-nerolidol (5%), alpha -cadinol (5.4%), and alpha-pinene (4.7%) were identified as main constituents of the essential oil. LC MS/MS analysis of the infusion and the methanol extract showed the presence of 15 phenolic compounds. Moreover, the total phenolic content of each sample was also determined and the infusion had the highest percentage of phenolics. To evaluate the antioxidant properties, the samples were tested by using DPPH" free radical scavenging, FRAP, and CUPRAC activity methods. The infusion showed the strongest radical scavenging activity, whereas n-hexane and chloroform extracts exhibited considerable reducing power effects. The MIC values for all of the examined microorganisms ranged from 15 to 2000 mug/mL with respect to antimicrobial activities.
A chemometric assessment of essential oil variation of three Salvia species indigenous to South Africa.[Pubmed:31958659]
Phytochemistry. 2020 Apr;172:112249.
Indigenous Salvia species from southern Africa are popular traditional medicines for the treatment of a variety of conditions. They produce fragrant volatiles that can be isolated as essential oils. Some of these volatile organic compounds may play a role in the biological activities of the extracts. Three indigenous Salvia species, Salvia africana-lutea, S. lanceolata and S. chamelaeagnea, were selected for this study as they are commonly used in traditional medicine in South Africa, and the essential oils from these species have potential for commercialisation. Although some studies have described the essential oil compositions and some biological activities, only single composite samples were used. The aim of this study was to investigate the intra- and interspecies variation of the essential oils, sampled over a wide geographical area and using a representative sample size, to encourage commercialisation of the essential oil. Essential oils were isolated from individual plants using conventional hydrodistillation of the aerial parts, harvested from several localities. Gas chromatography coupled simultaneously to mass spectrometry/flame ionisation detection (GC-MS/FID) was used to identify and quantify the volatile constituents. The essential oils of S. africana-lutea consisted mainly of terpinene-4-ol + beta-caryophyllene (1.4 - 29.0%), T-Cadinol (1.2 - 20.0%), alpha-eudesmol (trace - 23.0%) and beta-eudesmol (trace - 26.0%), those of S. lanceolata comprised mainly terpinene-4-ol + beta-caryophyllene (4.3 - 31.0%), alpha-humulene (2.3 - 15.0%), bicyclogermacrene (trace - 37.0%) and spathulenol (trace - 25.0%), while the essential oils of S. chamelaeagnea were characterised by delta-3-carene (trace - 18.0%), limonene (1.6 - 36.0%), viridiflorol (9.8 - 61.0%) and 1,8-cineole (not detected - 11.0%). The compounds identified in the essential oils of the three selected Salvia species have been identified in other Salvia essential oils. To add to the novelty of this study, the superior resolving power of two-dimensional gas chromatography was demonstrated through analysis of selected essential oils. Many additional compounds were identified, and previously co-eluting compounds were clearly separated. Chemometric modelling of the GC-MS data using SIMCA P+ 14 software allowed distinct clustering patterns to be discerned. The unsupervised principal component analysis model revealed separate clusters for the three species, confirming substantial chemical differences between their essential oils. Quantitative, rather than qualitative differences were evident when individual essential oil samples representing the same species, were compared. For each species, two chemically distinct groups were observed and unique marker compounds could be identified. This study has contributed detailed information on the major and minor volatile compounds present in the essential oils of the three Salvia species investigated.
A morphological, enzymatic and metabolic approach to elucidate apoptotic-like cell death in fungi exposed to h- and alpha-molybdenum trioxide nanoparticles.[Pubmed:30398279]
Nanoscale. 2018 Nov 15;10(44):20702-20716.
The present study compares for the first time the effects of h-MoO3 and alpha-MoO3 against two fungal strains: Aspergillus niger and Aspergillus flavus. The h-MoO3 nanoparticles were more toxic to both fungi than alpha-MoO3. The toxic effects of h-MoO3 were more pronounced toward A. flavus, which presented a growth inhibition of 67.4% at 200 mg L-1. The presence of the nanoparticles affected drastically the hyphae morphology by triggering nuclear condensation and compromising the hyphae membrane. Further analysis of the volatile organic compounds (VOCs) produced by both fungi in the presence of the nanomaterials indicated important metabolic changes related to programmed cell death. These nanomaterials induced the production of specific antifungal VOCs, such as beta-Elemene and T-Cadinol, by the fungi. The production of essential enzymes involved in fungal metabolism, such as acid phosphatase, naphthol-As-BI-phosphohydrolase, beta-galactosidase, beta-glucosidase and N-acetyl-beta-glucosaminidase, reduced significantly in the presence of the nanomaterials. The changes in enzymatic production and VOCs corroborate the fact that these nanoparticles, especially h-MoO3, exert changes in the fungal metabolism, triggering apoptotic-like cell death responses in these fungi.
Chemical composition, antioxidant, anticholinesterase, antimicrobial and antibiofilm activities of essential oil and methanolic extract of Anthemis stiparum subsp. sabulicola (Pomel) Oberpr.[Pubmed:29684540]
Microb Pathog. 2018 Jun;119:233-240.
Anthemis species are traditionally used to treat infectious and inflammatory processes, among others clinical disturbances. In the current study, the chemical composition, the total phenolic and flavonoid contents, the antioxidant, anticholinesterase, antimicrobial, and antibiofilm activities of Anthemis stiparum subsp. sabulicola aerial parts methanolic extract (As-ME) and essential oil (As-EO) were investigated. The chemical composition of As-EO was established by GC-MS and GC-FID. Total phenolic and flavonoid contents of As-ME were spectrophotometrically determined. Diphenyl-1-picrylhydrazyl (DPPH(●)) radical scavenging, cupric reducing antioxidant capacity (CUPRAC) and beta-carotene bleaching assays were applied to evaluate the antioxidant potential. The anticholinesterase activity against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes were carried out spectrophotometrically. The antimicrobial activity was assessed by Minimal Inhibitory Concentration (MIC) using broth microdilution method against 7 ATCC((R)) bacterial and one ATCC((R)) yeast reference strains. The antibiofilm effect was determined quantifying the percentage of adhesion inhibition. GC-MS and GC-FID identified 72 compounds (99.02%), being As-EO predominantly constituted by germacrene D (11.13%), T-Cadinol (11.01%), camphor (6.73%), spathulenol (6.50%) and isoamyl salicylate (6.45%). The total phenolic and flavonoid contents of As-ME were 13.6 +/- 0.03 and 5.9 +/- 0.04 pyrocatechol equivalents and quercetin equivalents, respectively. In beta-carotene-linoleic acid assay, As-ME showed the best lipid peroxidation inhibition activity with an IC(50) = 9.96 mug/mL followed by As-EO with an IC(50) = 619.98 mug/mL. In contrast, in DPPH assay, As-ME and As-EO showed moderate to low activity with an IC(50) = 92.69 mug/mL for As-ME and 917.69 mug/mL for As-EO. While in CUPRAC assay, As-EO and As-ME indicated a less to moderate reducing activity. As-ME inhibited AChE (IC(50) = 490.46 mug/mL) and BChE (IC(50) = 142.07 mug/mL), while As-EO was inactive against AChE and revealed a discreet inhibitory action against BChE (IC(50) = 212.14 mug/mL). As-ME displayed better antimicrobial activity than As-EO, being active against Staphylococcus aureus (ATCC((R)) 25923) and Bacillus subtilis (ATCC((R)) 6633), with MIC of 1.56 mg/mL. An expressive fungal adhesion inhibition (80.02%) on Candida albicans (ATCC((R)) 10239) was detected with As-ME at 6.25 mg/mL. These results showed that A. stiparum subsp. sabulicola is a natural source of active compounds with antibiotic and antibiofilm effects against S. aureus and B. subtilis, and C. albicans, respectively, and also presents antioxidant and anticholinesterase properties.
[Sesquiterpenes from stems of Schisandra henryi var. henryi].[Pubmed:28920347]
Zhongguo Zhong Yao Za Zhi. 2016 Aug;41(16):3049-3054.
The dried stems of Schisandra henryi var. henryi were extracted with 95% ethanol and the extracts were further subjected to partition, affording the ethyl acetate extracts(EtOAc Extrs.).The EtOAc Extrs.were separated and purified with silica gel and octadecyl-silylated silica gel column chromatography, preparative HPLC and preparative TLC. Thirteen known compounds were obtained and identified by spectral methods including MS and NMR, all of which were elucidated as T-Cadinol(1), cadinane-4beta,5alpha,10beta-triol(2), cadinane-5alpha, 10alpha-diol-2-ene(3), oxyphyllenodiols A(4), 1beta, 4beta-dihydroxyeudesman-11-ene(5), cyperusol C(6), (7R)-opposit-4(15)-ene-1beta,7-diol(7), dysodensiol E(8), epi-guaidiol A(9), aromadendrane-4beta,10beta-diol(10), tricyclohumuladiol(11), caryolane-1,9beta-diol(12), and guaidiol A(13). Compounds 3, 5-10, and 13 were separated from the genus for the first time, while compounds 1-13 were separated from this species for the first time.


