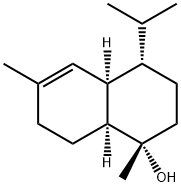
T-MuurololCAS# 19912-62-0 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 19912-62-0 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C15H26O | M.Wt | 206.41 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Synonyms | epi-α-Muurolol,T-Muurolol | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

T-Muurolol Dilution Calculator

T-Muurolol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8447 mL | 24.2236 mL | 48.4473 mL | 96.8945 mL | 121.1182 mL |
| 5 mM | 0.9689 mL | 4.8447 mL | 9.6895 mL | 19.3789 mL | 24.2236 mL |
| 10 mM | 0.4845 mL | 2.4224 mL | 4.8447 mL | 9.6895 mL | 12.1118 mL |
| 50 mM | 0.0969 mL | 0.4845 mL | 0.9689 mL | 1.9379 mL | 2.4224 mL |
| 100 mM | 0.0484 mL | 0.2422 mL | 0.4845 mL | 0.9689 mL | 1.2112 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Digitalin
Catalog No.:BCX2002
CAS No.:752-61-4
- 1,3-Dihydroxy-2-methoxyxanthone
Catalog No.:BCX2001
CAS No.:87339-74-0
- 1-Methoxy-2,3-methylenedioxyxanthone
Catalog No.:BCX2000
CAS No.:63625-05-8
- 1,2,3-Trimethoxyxanthone
Catalog No.:BCX1999
CAS No.:27460-10-2
- 3-Hydroxy-1,2-dimethoxyxanthone
Catalog No.:BCX1998
CAS No.:20362-27-0
- 5,6-Dihydroxy-8-methoxyflavone-7-O-glucuronide
Catalog No.:BCX1997
CAS No.:1169879-99-5
- (6S,9R)-2-Hydroxy-4-(2,6,6-trimethyl-4-oxo-cyclohex-2-enyl)-butyric acid
Catalog No.:BCX1996
CAS No.:2089289-59-6
- 5,6,7,8-Tetrahydroxy-3',4'-dimethoxyflavone
Catalog No.:BCX1995
CAS No.:2930091-30-6
- Anhydroperiplogenone
Catalog No.:BCX1994
CAS No.:1247-04-7
- Digitoxigenin glucomethyloside
Catalog No.:BCX1993
CAS No.:40950-57-0
- Strospeside
Catalog No.:BCX1992
CAS No.:595-21-1
- Quercetin-3-O-α-(6'''-caffeoylglucosyl-β-1,2-rhamnoside)
Catalog No.:BCX1991
CAS No.:851222-75-8
- T-Cadinol
Catalog No.:BCX2004
CAS No.:5937-11-1
- Paeonicluside
Catalog No.:BCX2005
CAS No.:448231-30-9
- Schiarisanrin D
Catalog No.:BCX2006
CAS No.:188943-88-6
- (-)-cis-Calamenene
Catalog No.:BCX2007
CAS No.:483-77-2
- (-)-Torreyol
Catalog No.:BCX2008
CAS No.:19435-97-3
- 17β-Tenacigenin B
Catalog No.:BCX2009
CAS No.:863767-79-7
- 2,5-Dihydroxy-7-methoxyflavanone
Catalog No.:BCX2010
CAS No.:35486-66-9
- 5,8-Dihydroxy-6,7,3′,4′-tetramethoxyflavone
Catalog No.:BCX2011
CAS No.:683278-67-3
- Ananonin A
Catalog No.:BCX2012
CAS No.:1314021-67-4
- Benzoyloxokadsuranol
Catalog No.:BCX2013
CAS No.:130252-47-0
- Heteroclitin F
Catalog No.:BCX2015
CAS No.:144049-67-2
- Tenuifoliose B
Catalog No.:BCX2016
CAS No.:139682-02-3
Essential oil composition, anti-tyrosinase activity, and molecular docking studies of Knema intermedia Warb. (Myristicaceae).[Pubmed:36960928]
Z Naturforsch C J Biosci. 2023 Mar 27;78(7-8):293-298.
Knema is one of the genera in the Myristicaceae family. The genus includes 60 species in Southeast Asia and is traditionally used for treating skin disorders. Here, for the first time, the essential oil, anti-tyrosinase, and molecular docking studies of Knema intermedia were evaluated. The essential oil was obtained by hydrodistillation and fully characterized by gas chromatography (GC-FID) and gas chromatography-mass spectrometry (GC-MS). Anti-tyrosinase activity was evaluated against mushroom tyrosinase, whereas molecular docking studies were performed using Autodock vina embedded in PyRx to evaluate the binding interactions of major components. A total of 37 components (97.3%) were successfully identified in the essential oil, which was characterized by high amounts of T-Muurolol (20.1%), alpha-copaene (14.4%), delta-cadinene (13.9%), germacrene B (9.5%), and delta-selinene (7.0%). The essential oil displayed moderate inhibitory activity towards tyrosinase with an IC(50) value of 70.2 mug/mL. The best docking energy was observed with delta-selinene (-7.8 kcal/mol), and it also forms interactions with His85, His263, and His244 which are important amino acid residues of the tyrosinase receptor. Hence, this study provides valuable scientific data on K. intermedia as potential candidate for the development of natural antiaging formulations.
Fumigant toxicity of four essential oils against the carob moth Ectomyelois ceratoniae Zeller and the Mediterranean flour moth Ephestia kuehniella.[Pubmed:36463575]
Int J Environ Health Res. 2024 Jan;34(1):419-431.
Eucalyptus leucoxylon, Rosmarinus officinalis, berries, and leaves of Schinus molle essential oils were analysed using GC-MS techniques and assessed for their fumigant toxicity against adults, larvae, and eggs of Ectomyelois ceratoniae and Ephestia kuehniella. Results showed that E. leucoxylon contained Spathulenol, p-Cymene, and Cryptone as major compounds. GC - MS analyses showed that the major compounds of R. officinalis essential oil were 1,8-Cineole, Camphor, and alpha-pinene. I-Phellandrene, T-Muurolol, and Phellandrene were the major components of the S. molle leaves; while I-Phellandrene, Limonen, and delta-Cadinene were the major components of the S. molle berries. S. molle berries were the most efficient followed by R. officinalis against E. ceratoniae and E. kuehniella. The most effective tested essential oil against E. ceratoniae larvae was S. molle berries. Results on the ovicidal and larvicidal effects of the tested oils demonstrated that R. officinalis was the most effective essential oil against E. kuehniella eggs.
Chemical composition and anticholinesterase activity of Lepisanthes rubiginosa (Roxb.) Leenh. essential oil.[Pubmed:36112782]
Z Naturforsch C J Biosci. 2022 Sep 19;77(11-12):525-529.
Essential oils obtained from medicinal plants show high therapeutic potential against several types of pathologies, including Alzheimer's disease. The purpose of this work was to study the chemical composition and anticholinesterase inhibitory activity of the essential oil obtained from Lepisanthes rubiginosa leaves collected from Malaysia. Twenty-four components were identified using gas chromatography-flame ionization detection (GC-FID) and gas chromatography/mass spectrometry (GC-MS), which represent 99.5% of the essential oil. The identified major components include alpha-cadinol (40.0%), safrole (12.6%), alpha-amorphene (9.5%), (E)-isosafrole (5.0%), delta-cadinene (4.2%), and T-Muurolol (4.1%). Anticholinesterase activity was assessed using Ellman method, and the essential oil demonstrated a moderate inhibitory activity against acetylcholinesterase (I%: 75.2%) and butyrylcholinesterase (I%: 70.2%) at conconcetration of 1000 mug/mL. The current study is the first to report chemical composition and anticholinesterase activity of the essential oil obtained from L. rubiginosa, which may have implications on the characterization, pharmaceutical, and therapeutic applications of Lepisanthes genus essential oils.
Hirtellina lobelii DC. essential oil, its constituents, its combination with antimicrobial drugs and its mode of action.[Pubmed:30654130]
Fitoterapia. 2019 Mar;133:130-136.
With the goal of unravelling antimicrobial agents and mixtures inspired by plant defences, we investigated the antibacterial and antifungal efficacy of Hirtellina lobelii DC. essential oil (EO), both alone and in combination with antimicrobial drugs. Hirtellina lobelii DC. EO was analysed by GC, GC-MS and partial fractionation/NMR. It was essentially composed of oxygenated sesquiterpenes (75.2%), with alpha-bisabolol (34.5%), fokienol (12.0%) and T-Muurolol (6.8%) serving as the main components. Microbial susceptibility was determined by the broth microdilution method and was expressed as minimum inhibitory concentration (MIC) and minimum bactericidal or fungicidal concentration (MBC or MFC). This EO was found to possess remarkable bactericidal (MBC/MIC = 2) and fungicidal (MFC/MIC = 1-4) potential, particularly against the Gram (+) bacteria Staphylococcus aureus, including its methicillin-resistant forms, the yeast Cryptococcus neoformans and dermatophytes from the genus Trichophyton (MICs 8-128 mug/ml). The examination of the combined effects of the EO with antimicrobial drugs revealed synergisms of the EO with vancomycin against S. aureus and of the EO with fluconazole and griseofulvin against dermatophytic fungi (FICI 0.2-0.5). The effect of H. lobelii EO on the morphologies of fungal hyphae and bacteria, as determined by scanning electronic microscopy (SEM), showed fungal hyphae swelling and bulging. These results suggest that H. lobelii EO and its major constituent, alpha-bisabolol, have remarkable antimicrobial potential. Combination therapies of this EO with antifungal drugs could offer a promising alternative for treatment of human mycoses caused by filamentous dermatophytic fungi.
An Isotopic Labelling Strategy to Study Cytochrome P450 Oxidations of Terpenes.[Pubmed:29697903]
Chembiochem. 2018 Jul 16;19(14):1498-1501.
The cytochrome P450 monooxygenase CYP267B1 from Sorangium cellulosum was applied for the enzymatic oxidation of the sesquiterpene alcohols T-Muurolol and isodauc-8-en-11-ol. Various isotopically labelled geranyl and farnesyl diphosphates were used for product identification from micro-scale reactions, for the determination of the absolute configurations of unknown compounds, to follow the stereochemical course of a cytochrome P450-catalysed hydroxylation step, and to investigate kinetic isotope effects. Overall, this study demonstrates that isotopically labelled terpene precursors are highly useful to follow cytochrome P450 dependent oxidations of terpenes.
[Sesquiterpenoids from rhizome of Homalomena occulta].[Pubmed:28905602]
Zhongguo Zhong Yao Za Zhi. 2016 Jul;41(14):2655-2659.
Twelve compounds were isolated from alcohol extracts of the rhizome of Homalomena occulta by using various chromatographic techniques including column chromatography onsilica gel and C(1)(8) reverse-phase silica gel, and semi-preparative HPLC. Their structures were identified by physico-chemical properties and spectroscopic data analysis as 3alpha, 7alpha-dihydroxy-cadin-4-ene (1), 3-oxofabiaimbricatan (2), 3beta, 4alpha-dihydroxy-7-epi-eudesm-11(13)-ene (3), integrifonol A(4), 1beta, 6beta-dihydroxy-7-epi-eudesm-11(13)-ene (5), 4beta, 7beta, 11-enantioeudesmantriol (6), epi-guaidiol (7), oplopanone(8), (-)-1beta, 4beta, 6alpha-trihydroxy-eudesmane (9),2alpha-hydroxyhomalomenol(10), (-)-T-Muurolol (11) and hamalomenol A(12). Compounds 1-7 were obtained from the genus Homalomena for the first time and 11-12 were firstly reported from the species. Additionally, compounds 3, 5 and 8 displayed inhibitory effects against the lipopolysaccharide (LPS)-induced nitric oxide (NO) production in mouse macrophage RAW264.7 cells with IC(5)(0) values of 6.51, 3.25, 7.78 mumol*L(-)(1), respectively.
Isolation and absolute configurations of diastereomers of 8alpha-hydroxy-T-muurolol and (1alpha,6beta,7beta)-cadinane-4-en-8alpha,10alpha-diol from Chimonanthus salicifolius.[Pubmed:26790964]
Phytochemistry. 2016 Feb;122:294-300.
Phytochemical investigation of the aerial parts of Chimonanthus salicifolius resulted in the isolation of two sesquiterpenoids, 8alpha-hydroxy-T-Muurolol and (1alpha,6beta,7beta)-cadinane-4-en-8alpha,10alpha-diol, together with 13 known compounds. The 15 structures were established by means of 1D and 2D NMR spectroscopy. The relative and absolute configurations of 8alpha-hydroxy-T-Muurolol and 8alpha,11-elemodiol were achieved by NOESY experiments and X-ray crystallography using CuKalpha radiation. 8alpha-hydroxy-T-Muurolol and (1alpha,6beta,7beta)-cadinane-4-en-8alpha,10alpha-diol showed immunosuppressive activities in a dose-dependent manner.
[Chemical constituents of leaves of Psidium guajava].[Pubmed:24956844]
Zhongguo Zhong Yao Za Zhi. 2014 Mar;39(6):1024-9.
To study the chemical constituents of the 95% ethanol extract of Psidium guajava. Compounds were separated by using a combination of various chromatographic methods including silica gel, D101 macroporous resin, ODS, Sephadex LH-20 and preparative HPLC. Their structures were elucidated by physicochemical properties and spectral data Eighteen compounds were isolated and identified as (+) -globulol (1), clovane-2beta, 9alpha-diol (2), 2beta-acetoxyclovan-9alpha-ol (3), (+) -caryolane-1 ,9beta-diol (4), ent-T-Muurolol (5), clov-2-ene-9alpha-ol (6), isophytol (7), tamarixetin (8), gossypetin (9), quercetin (10), kaempferol (11), guajaverin (12), avicularin (13), chrysin 6-C-glucoside (14), 3'-O-methyl-3, 4-methylenedioxyellagic acid 4'-O-beta-D-glucopyranoside (15), p-hydroxy-benzoic acid (16), guavinoside A (17) and guavinoside B (18). Compounds 2-9 and 14-16 were isolated from this plant for the first time. The ethanol extract showed 61.3% inhibition against the proliferation of colon cancer cell line SW480.
Essential oils of Chiliadenus lopadusanus (Asteraceae).[Pubmed:24079193]
Nat Prod Commun. 2013 Aug;8(8):1159-62.
The essential oils from the leaves and flowers of Chiliadenus lopadusanus growing on Lampedusa Island were obtained by hydrodistillation and analyzed by GC-MS. The major component was camphor (39.4% in the leaves and 24.0% in the flowers), followed in the leaves by torreyol (6.7%), t-cadinol (5.2%) and 1,8-cineole (3.8%), while in the flowers by t-cadinol (15.2%), T-Muurolol (5.1%) and torreyol (4.5%). Among the compounds identified, several seem to play a role in antibacterial, antifungal, allelopathic and spasmolytic activity. In addition, several compounds identified in this study seem to influence the attraction of Megachile (Eutricharaea) apicalis (Megachilidae) and Halictus (Seladonia) gemmeus (Halictidae), two hymenopteran here identified as pollinators of Chiliadenus lopadusanus.
Nudibaccatumone, a trimer comprising a phenylpropanoid and two sesquiterpene moieties from Piper nudibaccatum.[Pubmed:23544451]
J Nat Prod. 2013 Apr 26;76(4):732-6.
A new complex natural product with a C39 skeleton, named nudibaccatumone, and the known sesquiterpenes (+)-spathulenol, (-)-4beta,10alpha-aromadendranediol, and ent-T-Muurolol, as well as the phenylpropanoid hydroxychavicol, were isolated from the aerial parts of Piper nudibaccatum. The structure and absolute configuration of nudibaccatumone were elucidated using spectroscopic methods and ECD calculations. A 1,8-Michael addition reaction and an intermolecular, inverse electron demand Diels-Alder reaction are proposed as the key steps in the biosynthesis of nudibaccatumone.
[Sesquiterpenes from stem of Schisandra glaucescens].[Pubmed:23373215]
Zhongguo Zhong Yao Za Zhi. 2012 Nov;37(22):3426-9.
OBJECTIVE: To study the chemical constituents from Schisandra glaucescens. METHOD: The chemical constituents were separated and purifed with silica gel, gel column chromatography preparative HPLC, and their structures were identified by such spectral methods as MS and NMR. RESULT: Twelve compounds were separated from petroleum ether fractions, and identified as t-cadinol (1), alpha-cadinol (2), torreyol (3), (+)-ent-epicubenol (4), ent-T-Muurolol (5), (-)-15-hydroxycalamenene (6), (-)-cubebol (7), 4-epi-cubebol (8), caryophyllenol-I (9), caryophyllenol-II (10), oxyphyllenodiols A (11), caryolane-1,9/3-diol (12). CONCLUSION: Compounds 4, 6-12 were separated from the genus for the first time, while compounds 1-12 were separated from this plant for the first time.
Bioactive constituents of Homalomena aromatica essential oil and its antifungal activity against dermatophytes and yeasts.[Pubmed:23177818]
J Mycol Med. 2012 Mar;22(1):83-7.
Homalomena aromatica rhizomes are rich source of essential oils, which have been attributed for various medicinal uses. In the present investigation, essential oil from H. aromatica rhizomes was isolated and subjected to gas chromatography-mass spectrum (GC-MS) analysis. Fifty-five chemical constituents were reported from H. aromatica rhizomes of which T-Muurolol (5.32%), viridiflorol (3.69%), alpha-selinene (2.19%), M-cymene (2.19%) and gamma-Muurolene (1.81%) were identified and reported for the first time. Other major components were identified as linalool (62.5%), terpene-4-ol (7.08%), delta-cadinene (5.57%), alpha-cadinol (3.71%) and spatulenol (1.81%). H. aromatica rhizome essential oil showed high antimicrobial activity against Trichophyton rubrum, Trichophyton mentagrophytes, Microsporum fulvum, Microsporum gypseum, Trichosporon beigelii and Candida albicans.
Antioxidant and antimicrobial activities of essential oil and extracts of Saurauia lantsangensis hu root.[Pubmed:22888533]
Z Naturforsch C J Biosci. 2012 May-Jun;67(5-6):282-90.
Antioxidant and antimicrobial activities of the essential oil and n-hexane (HEE), chloroform (CHE), ethyl acetate (EAE), and methanol (MEE) extracts, respectively, from the root of Saurauia lantsangensis Hu were investigated. The GC-MS analysis revealed 39 compounds representing 96.41% of the oil containing T-Muurolol (13.85%), acetophenone (7.46%), alpha-cadinol (6.26%), methyl palmitate (5.36%), n-hexadecanoic acid (4.31%), torreyol (3.69%), and isospathulenol (3.48%) as major components. Antioxidant activities determined by three various testing systems, i.e., DPPH radical scavenging, superoxide anion radical scavenging, and reducing power assay, increased in the order: HEE < CHE < oil < MEE < EAE. CHE, EAE, MEE and oil exhibited a promising antimicrobial effect determined as the diameter of zones of inhibition (13.3-16.2, 16.5-20.4, 13.5-16.6, and 16.5-22.7 mm), respectively, along with their respective MIC values (500-1000, 125-500, 250-500, and 250-500 microg/ml) against Gram-negative bacteria (Pseudomonas aeruginosa, Escherichia coli), Gram-positive bacteria (Bacillus subtilis, Staphylococcus aureus), and a yeast (Hansenula anomala).


