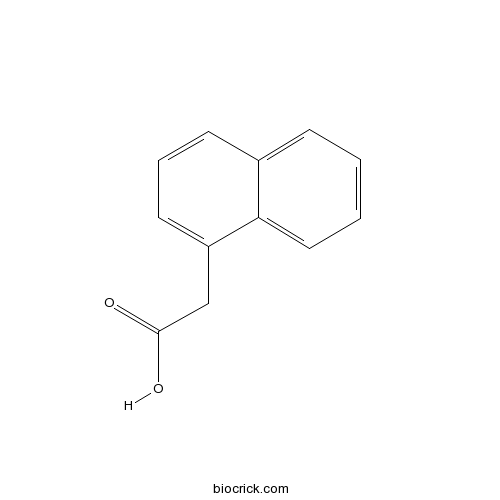
1-Naphthaleneacetic acidCAS# 86-87-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 86-87-3 | SDF | Download SDF |
| PubChem ID | 6862 | Appearance | Powder |
| Formula | C12H10O2 | M.Wt | 186.21 |
| Type of Compound | Other Compounds | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-naphthalen-1-ylacetic acid | ||
| SMILES | C1=CC=C2C(=C1)C=CC=C2CC(=O)O | ||
| Standard InChIKey | PRPINYUDVPFIRX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H10O2/c13-12(14)8-10-6-3-5-9-4-1-2-7-11(9)10/h1-7H,8H2,(H,13,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

1-Naphthaleneacetic acid Dilution Calculator

1-Naphthaleneacetic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.3703 mL | 26.8514 mL | 53.7028 mL | 107.4056 mL | 134.257 mL |
| 5 mM | 1.0741 mL | 5.3703 mL | 10.7406 mL | 21.4811 mL | 26.8514 mL |
| 10 mM | 0.537 mL | 2.6851 mL | 5.3703 mL | 10.7406 mL | 13.4257 mL |
| 50 mM | 0.1074 mL | 0.537 mL | 1.0741 mL | 2.1481 mL | 2.6851 mL |
| 100 mM | 0.0537 mL | 0.2685 mL | 0.537 mL | 1.0741 mL | 1.3426 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isopongachromene
Catalog No.:BCN9034
CAS No.:
- Cichoric acid
Catalog No.:BCN9033
CAS No.:6537-80-0
- Anethole, trans-
Catalog No.:BCN9032
CAS No.:4180-23-8
- Galactaric acid
Catalog No.:BCN9031
CAS No.:526-99-8
- (±)-Nicotine
Catalog No.:BCN9030
CAS No.:22083-74-5
- Cynatratoside C
Catalog No.:BCN9029
CAS No.:
- Byakangelicin
Catalog No.:BCN9028
CAS No.:482-25-7
- Neolancerin
Catalog No.:BCN9027
CAS No.:117221-65-5
- (-)-Carvone
Catalog No.:BCN8949
CAS No.:6485-40-1
- Cyanidin-3-O-(6''-malonylglucoside) chloride
Catalog No.:BCN9026
CAS No.:171828-62-9
- Sipeimine-3-beta-D-glucoside
Catalog No.:BCN9025
CAS No.:67968-40-5
- Robinetinidin chloride
Catalog No.:BCN9024
CAS No.:3020-09-5
- Kizuta saponin K11
Catalog No.:BCN9036
CAS No.:97240-03-4
- D-(+)-Lactose Monohydrate
Catalog No.:BCN9037
CAS No.:64044-51-5
- Ganoderic acid E
Catalog No.:BCN9038
CAS No.:
- Fluoranthene
Catalog No.:BCN9039
CAS No.:206-44-0
- DL-α-Tocopherol
Catalog No.:BCN9040
CAS No.:10191-41-0
- Peimisine hydrochloride
Catalog No.:BCN9041
CAS No.:900498-44-4
- (+)-Secoisolariciresinol
Catalog No.:BCN9042
CAS No.:145265-02-7
- Allocryptopine
Catalog No.:BCN9043
CAS No.:485-91-6
- Quininic acid
Catalog No.:BCN9044
CAS No.:86-68-0
- Menthol
Catalog No.:BCN9045
CAS No.:89-78-1
- Atractyloside potassium salt
Catalog No.:BCN9046
CAS No.:17754-44-8
- Deslanoside
Catalog No.:BCN9047
CAS No.:17598-65-1
YUCCA-Mediated Biosynthesis of the Auxin IAA Is Required during the Somatic Embryogenic Induction Process in Coffea canephora.[Pubmed:32635392]
Int J Mol Sci. 2020 Jul 3;21(13). pii: ijms21134751.
Despite the existence of considerable research on somatic embryogenesis (SE), the molecular mechanism that regulates the biosynthesis of auxins during the SE induction process remains unknown. Indole-3-acetic acid (IAA) is an auxin that is synthesized in plants through five pathways. The biosynthetic pathway most frequently used in this synthesis is the conversion of tryptophan to indol-3-pyruvic acid (IPA) by tryptophan aminotransferase of Arabidopsis (TAA) followed by the conversion of IPA to IAA by enzymes encoded by YUCCA (YUC) genes of the flavin monooxygenase family; however, it is unclear whether YUC-mediated IAA biosynthesis is involved in SE induction. In this study, we report that the increase of IAA observed during SE pre-treatment (plants in MS medium supplemented with 1-Naphthaleneacetic acid (NAA) 0.54 microM and kinetin (Kin) 2.32 microM for 14 days) was due to its de novo biosynthesis. By qRT-PCR, we demonstrated that YUC gene expression was consistent with the free IAA signal found in the explants during the induction of SE. In addition, the use of yucasin to inhibit the activity of YUC enzymes reduced the signal of free IAA in the leaf explants and dramatically decreased the induction of SE. The exogenous addition of IAA restored the SE process in explants treated with yucasin. Our findings suggest that the biosynthesis and localization of IAA play an essential role during the induction process of SE in Coffea canephora.
The regeneration of Acer rubrum L. "October Glory" through embryonic callus.[Pubmed:32615933]
BMC Plant Biol. 2020 Jul 2;20(1):309.
BACKGROUND: Tissue culture and rapid propagation technology is an important way to solve the difficulties of plant propagation. This experiment aims to explore the appropriate conditions at each stage of the red maple's tissue culture process and to obtain plantlets, thus providing a theoretical basis for the establishment of the red maple's tissue culture system. RESULTS: The results showed that the stem segment is the most suitable explant for inducing embryogenic callus. The MS (Murashige&Skoog) + 0.8 mg/L TDZ (Thidiazuron) + 1.0 mg/L 6-BA (6-Benzylaminopurine) + 0.5 mg/L IAA(Indole-3-acetic acid) + 35 g/L sucrose+ 7.5 g/L semi-fixed medium was the best for callus formation. When selecting type VI callus as embryonic callus induction material, MS + 0.6 mg/L TDZ + 0.5 mg/L 6-BA + 2.0 mg/L IAA + 35 g/L sucrose+ 7.5 g/L semi-fixed medium can get embryonic callus. The optimal medium for adventitious bud induction is MS + 1.0 mg/L TDZ + 3.0 mg/L 6-BA+ 0.2 mg/L NAA (1-Naphthaleneacetic acid) + 1.2 mg/L IAA + 35 g/L sucrose+ 7.5 g/L semi-fixed medium. The induction rate of adventitious roots in MS + 0.6 mg/L TDZ + 1.0 mg/L 6-BA+ 3 mg/L NAA + 35 g/L sucrose+ 7.5 g/L semi-fixed medium was the highest, reaching 76%. CONCLUSIONS: In the course of our research, we found that PGRs play an important role in the callus induction stage, and the effect of TDZ is particularly obvious; The callus cells grow and proliferate according to the "S" growth curve, and can be sub-cultured when the highest growth point is reached to maintain the rapid proliferation of the callus cells and to avoid inactivation of callus caused by tight niche.
Genome-wide characterization and expression analyses of the auxin/indole-3-acetic acid (Aux/IAA) gene family in barley (Hordeum vulgare L.).[Pubmed:32581321]
Sci Rep. 2020 Jun 24;10(1):10242.
Aux/IAA genes are early auxin-responsive genes and essential for auxin signaling transduction. There is little information about Aux/IAAs in the agriculturally important cereal, barley. Using in silico method, we identified and subsequently characterized 36 Aux/IAAs from the barley genome. Based on their genomic sequences and the phylogenic relationship with Arabidopsis and rice Aux/IAA, the 36 HvIAAs were categorized into two major groups and 14 subgroups. The indication of the presence or absence of these domains for the biological functions and acting mechanisms was discussed. The cis-element distributions in HvIAA promoters suggests that the HvIAAs expressions may not only regulated by auxin (the presence of AuxREs and TGA-element) but also by other hormones and developmental and environmental cues. We then studied the HvIAAs expression in response to NAA (1-Naphthaleneacetic acid) using quantitative real-time PCR (qRT-PCR). Like the promoter analysis, only 14 HvIAAs were upregulated by NAA over two-fold at 4 h. HvIAAs were clustered into three groups based on the spatiotemporal expression data. We confirmed by qRT-PCR that most HvIAAs, especially HvIAA3, HvIAA7, HvIAA8, HvIAA18, HvIAA24 and HvIAA34, are expressed in the developing barley spike compared within seedling, suggesting their roles in regulating spike development. Taken together, our data provide a foundation for further revealing the biological function of these HvIAAs.
Influence of photoperiod on growth, bioactive compounds and antioxidant activity in callus cultures of Basella rubra L.[Pubmed:32570057]
J Photochem Photobiol B. 2020 Jun 14;209:111937.
Basella rubra L. is an important green leafy vegetable vine and is known for its health benefits in traditional medicine. Light is a basic physical factor essential to the development and bioactive secondary metabolite production in in vitro callus cultures. The present study researched the impact of different photoperiods on biomass, bioactive compounds, and antioxidant activity in callus cultures of B. rubra. The in vitro seedling based cotyledonary leaf explants responded differently, when cultured on Murashige and Skoog (MS) medium with varying concentrations and combination of auxins and cytokinins. The best callus proliferation was found in MS medium with 0.1 mg.L(-1) 1-Naphthaleneacetic acid (NAA) and 6 mg.L(-1) 6-benzylaminopurine (BAP), with greenish callus inception by about 2 weeks. The growth curve recorded for 6 weeks of culturing revealed that the photoperiod effect was found to be pivotal for acquiring biomass. At the fifth week, the continuous light supported maximum biomass (12.42 g) production followed by the 16:8 h photoperiod (9.02 g) and continuous darkness (4.28 g). The 80% ethanol extract of 1-week-old callus that grows under the 16:8 h photoperiod showed the highest total phenolic content (TPC) (74 mg.100 g(-1) fresh weight, FW) when compared to all other extracts at different stages. The ferric reducing antioxidant power assay showed the highest (336.23 mg.100 g(-1) FW) activity in methanol extractions of first-week callus cultures maintained in the continuous light condition. HPLC-UV identification and quantification of individual phenolics and flavonoids, such as gallic, trans-cinnamic, quercetin, protocatechuic and rutin, were highest in the callus cultures. The outcome of this study is significant to this plant, as B. rubra is familiar for its important health constituents with high-value bioactives and applications in the pharma and nutraceutical industries.
Effects of Biogenic Zinc Oxide Nanoparticles on Growth and Oxidative Stress Response in Flax Seedlings vs. In Vitro Cultures: A Comparative Analysis.[Pubmed:32560534]
Biomolecules. 2020 Jun 17;10(6). pii: biom10060918.
Linum usitatissimum biosynthesizes lignans and neolignans that are diet and medicinally valuable metabolites. In recent years, zinc oxide nanoparticles (ZnONPs) have emerged as potential elicitors for the enhanced biosynthesis of commercial secondary metabolites. Herein, we investigated the influence of biogenic ZnONPs on both seedlings and stem-derived callus of L. usitatissimum. Seedlings of L. usitatissimum grown on Murashige and Skoog (MS) medium supplemented with ZnONPs (1-1000 mg/L) presented the highest antioxidant activity, total phenolic content, total flavonoid content, peroxidase and superoxide dismutase activities at 500 mg/L, while the maximum plantlet length was achieved with 10 mg/L. Likewise, the high-performance liquid chromatography (HPLC) analysis revealed the enhanced production of secoisolariciresinol diglucoside, lariciresinol diglucoside, dehydrodiconiferyl alcohol glucoside and guaiacylglycerol-beta-coniferyl alcohol ether glucoside in the plantlets grown on the 500 mg/L ZnONPs. On the other hand, the stem explants were cultured on MS media comprising 1-Naphthaleneacetic acid (1 mg/L) and ZnONPs (1-50 mg/L). The highest antioxidant and other activities with an enhanced rooting effect were noted in 25 mg/L ZnONP-treated callus. Similarly, the maximum metabolites were also accumulated in 25 mg/L ZnONP-treated callus. In both systems, the dose-dependent production of reactive oxygen species (ROS) was recorded, resulting in oxidative damage with a more pronounced toxic effect on in vitro cultures. Altogether, the results from this study constitute a first comprehensive view of the impact of ZnONPs on the oxidative stress and antioxidant responses in seedlings vs. in vitro cultures.
Different Roles of Auxins in Somatic Embryogenesis Efficiency in Two Picea Species.[Pubmed:32403374]
Int J Mol Sci. 2020 May 11;21(9). pii: ijms21093394.
The effects of auxins 2,4-D (2,4-dichlorophenoxyacetic acid), NAA (1-Naphthaleneacetic acid) or picloram (4-amino-3,5,6-trichloropicolinic acid; 9 microM) and cytokinin BA (benzyloadenine; 4.5 microM) applied in the early stages of somatic embryogenesis (SE) on specific stages of SE in Picea abies and P. omorika were investigated. The highest SE initiation frequency was obtained after 2,4-D application in P. omorika (22.00%) and picloram application in P. abies (10.48%). NAA treatment significantly promoted embryogenic tissue (ET) proliferation in P. abies, while 2,4-D treatment reduced it. This reduction was related to the oxidative stress level, which was lower with the presence of NAA in the proliferation medium and higher with the presence of 2,4-D. The reduced oxidative stress level after NAA treatment suggests that hydrogen peroxide (H2O2) acts as a signalling molecule and promotes ET proliferation. NAA and picloram in the proliferation medium decreased the further production and maturation of P. omorika somatic embryos compared with that under 2,4-D. The quality of the germinated P. abies embryos and their development into plantlets depended on the auxin type and were the highest in NAA-originated embryos. These results show that different auxin types can generate different physiological responses in plant materials during SE in both spruce species.
Coumarin-Caged Compounds of 1-Naphthaleneacetic Acid as Light-Responsive Controlled-Release Plant Root Stimulators.[Pubmed:32396350]
J Agric Food Chem. 2020 Jun 10;68(23):6268-6279.
Six coumarin-caged compounds of 1-Naphthaleneacetic acid (NAA) comprising different substituents on the coumarin moiety were synthesized and evaluated for their photophysical and chemical properties as light-responsive controlled-release plant root stimulators. The (1)H NMR and HPLC techniques were used to verify the release of NAA from the caged compounds. After irradiation at 365 nm, the caged compounds exhibited the fastest release rate at t1/2 of 6.7 days and the slowest release rate at t1/2 of 73.7 days. Caged compounds at high concentrations (10(-5) and 10(-6) M) significantly stimulate secondary root germination while free NAA at the same level is toxic and leads to inhibition of secondary root germination. The cytotoxicity of the caged compounds against fibroblasts and vero cells were evaluated, and the results suggested that, at 10(-5)-10(-6) M, caged compounds exhibited no significant cytotoxicity to the cells. Thus, the caged compounds of NAA in this study could be of great benefit as efficient agrochemicals.
An efficient in vitro regeneration system from different wild apple (Malus sieversii) explants.[Pubmed:32336979]
Plant Methods. 2020 Apr 21;16:56.
Background: Wild apple, Malus sieversii, is an endangered species and a valuable genetic resource that requires a variety of conservation techniques. This study aimed to investigate the influence of different concentrations of hormones on wild apple regeneration from leaf and stem explants to establish an optimal regeneration system. Results: Leaves and stems derived from seedlings were cultured on several media supplemented with various concentrations of thidiazuron (TDZ) or 6-benzylaminopurine (BA) in different combinations with 1-Naphthaleneacetic acid (NAA). The results showed that the most efficient shoot formation media (35% and 90%) were MS medium containing 4.0 mg L(-1) TDZ and 1.0 mg L(-1) NAA for leaf explants and MS medium containing 1.0 mg L(-1) BA without NAA for stem explant. MS medium supplemented with 0.4 mg L(-1) BA and 0.1 mg L(-1) NAA (for shoot multiplication) and 1/2 MS + 0.1 mg L(-1) NAA + 1.5% sucrose (for rooting) were effective media. Shoot regeneration from leaf explants was the most effective when the explants were placed abaxial side down onto the medium and were subjected to a pre-treatment of 3 weeks in darkness. Conclusions: An optimized regeneration system for M. sieversii that allowed regeneration within 2-3 months developed. The protocol developed herein can be used in large-scale clonal propagation for the conservation of wild apple, M. sieversii.
In vitro production and distribution of flavonoids in Glycyrrhiza uralensis Fisch.[Pubmed:32180652]
J Food Sci Technol. 2020 Apr;57(4):1553-1564.
Glycyrrhiza uralensis Fisch. is known as a common Chinese medicinal herb used to harmonize the effects of other ingredients in most Chinese herbal prescriptions. The rapid production of flavonoids in vitro remains unknown in G. uralensis Fisch. To investigate the in vitro adventitious root regeneration and flavonoid accumulation characteristics in G. uralensis for restrictions on collecting wild plants, suspension cultural and freezing microtomy with histochemical assays were carried out. We reported that multiple adventitious roots were initiated from hypocotyls and stems of G. uralensis. Indole-3-butyric acid (IBA) was more conducive than NAA (1-Naphthaleneacetic acid) in inducing G. uralensis adventitious roots, but the addition of 6-BA (6-benzylaminopurine) and KT (kinetin) suppressed the formation of adventitious roots. While the concentration of IBA was 1.0 mg L(-1), the flavonoid content and yield were the highest at 19.96 mg g(-1) and 1.23 mg g(-1), respectively. The optimum medium for adventitious root induction was 1/4-strength Murashige and Skoog's medium containing 0.1 mg L(-1) IBA. The content of flavonoids in adventitious roots and apicals cultured in vitro was higher than that in suspension callus, reaching 3.87 times the callus flavonoid content. The histochemical localization of flavonoids showed that G. uralensis flavonoids mainly distributed in the epidermal parenchyma cells of the callus outer layers and gradually accumulated in cell wall and cell gaps of the epidermis and endodermis of adventitious roots along with the primary growth of adventitious roots, indicating that there were no flavonoids in the roots at the early stage of adventitious roots formation. The results showed that calli inducing adventitious roots and apicals for 30 days obtained the highest yield of flavonoid, indicating effective production for flavonoids instead of wild culture. AlCl3 ethanol solution was better than NaOH aqueous solution in terms of chromogenic and localization effects. We concluded that the highest yield of flavonoid and effective production for flavonoid instead of wild culture could be obtained from calli inducing adventitious roots and apicals.
Establishment of an Agrobacterium mediated transformation protocol for the detection of cytokinin in the heterophyllous plant Hygrophila difformis (Acanthaceae).[Pubmed:32146519]
Plant Cell Rep. 2020 Jun;39(6):737-750.
KEY MESSAGE: This is the first report of a highly efficient Agrobacterium tumefaciens-mediated transformation protocol for Acanthaceae and its utilization in revealing important roles of cytokinin in regulating heterophylly in Hygrophila difformis. Plants show amazing morphological differences in leaf form in response to changes in the surrounding environment, which is a phenomenon called heterophylly. Previous studies have shown that the aquatic plant Hygrophila difformis (Acanthaceae) is an ideal model for heterophylly study. However, low efficiency and poor reproducibility of genetic transformation restricted H. difformis as a model plant. In this study, we reported successful induction of callus, shoots and the establishment of an efficient stable transformation protocol as mediated by Agrobacterium tumefaciens LBA4404. We found that the highest callus induction efficiency was achieved with 1 mg/L 1-Naphthaleneacetic acid (NAA) and 2 mg/L 6-benzyladenine (6-BA), that efficient shoot induction required 0.1 mg/L NAA and 0.1 mg/L 6-BA and that high transformation efficiency required 100 microM acetosyringone. Due to the importance of phytohormones in the regulation of heterophylly and the inadequate knowledge about the function of cytokinin (CK) in this process, we analyzed the function of CK in the regulation of heterophylly by exogenous CK application and endogenous CK detection. By using our newly developed transformation system to detect CK signals, contents and distribution in H. difformis, we revealed an important role of CK in environmental mediated heterophylly.
NAA and 6-BA promote accumulation of oleanolic acid by JA regulation in Achyranthes bidentata Bl.[Pubmed:32107496]
PLoS One. 2020 Feb 27;15(2):e0229490.
Application of plant growth regulators has become one of the most important means of improving yield and quality of medicinal plants. To understand the molecular basis of phytohormone-regulated oleanolic acid metabolism, RNA-seq was used to analyze global gene expression in Achyranthes bidentata treated with 2.0 mg/L 1-Naphthaleneacetic acid (NAA) and 1.0 mg/L 6-benzyladenine (6-BA). Compared with untreated controls, the expression levels of 20,896 genes were significantly altered with phytohormone treatment. We found that 13071 (62.5%) unigenes were up-regulated, and a lot of differentially expressed genes involved in hormone or terpenoid biosynthesis, or transcription factors were significantly up-regulated. These results suggest that oleanolic acid biosynthesis induced by NAA and 6-BA occurs due to the expression of key genes involved in jasmonic acid signal transduction. This study is the first to analyze the production and hormonal regulation of medicinal A. bidentata metabolites at the molecular level. The results herein contribute to a better understanding of the regulation of oleanane-type triterpenoid saponins accumulation and define strategies to improve the yield of these useful metabolites.
Osmotic stress inhibits leaf growth of Arabidopsis thaliana by enhancing ARF-mediated auxin responses.[Pubmed:32077108]
New Phytol. 2020 Jun;226(6):1766-1780.
We investigated the interaction between osmotic stress and auxin signaling in leaf growth regulation. Therefore, we grew Arabidopsis thaliana seedlings on agar media supplemented with mannitol to impose osmotic stress and 1-Naphthaleneacetic acid (NAA), a synthetic auxin. We performed kinematic analysis and flow-cytometry to quantify the effects on cell division and expansion in the first leaf pair, determined the effects on auxin homeostasis and response (DR5::beta-glucuronidase), performed a next-generation sequencing transcriptome analysis and investigated the response of auxin-related mutants. Mannitol inhibited cell division and expansion. NAA increased the effect of mannitol on cell division, but ameliorated its effect on expansion. In proliferating cells, NAA and mannitol increased free IAA concentrations at the cost of conjugated IAA and stimulated DR5 promotor activity. Transcriptome analysis shows a large overlap between NAA and osmotic stress-induced changes, including upregulation of auxin synthesis, conjugation, transport and TRANSPORT INHIBITOR RESPONSE1 (TIR1) and AUXIN RESPONSE FACTOR (ARF) response genes, but downregulation of Aux/IAA response inhibitors. Consistently, arf7/19 double mutant lack the growth response to auxin and show a significantly reduced sensitivity to osmotic stress. Our results show that osmotic stress inhibits cell division during leaf growth of A. thaliana at least partly by inducing the auxin transcriptional response.
Auxin treatment of grapevine (Vitis vinifera L.) berries delays ripening onset by inhibiting cell expansion.[Pubmed:32043226]
Plant Mol Biol. 2020 May;103(1-2):91-111.
KEY MESSAGE: Auxin treatment of grape (Vitis vinifera L.) berries delays ripening by inducing changes in gene expression and cell wall metabolism and could combat some deleterious climate change effects. Auxins are inhibitors of grape berry ripening and their application may be useful to delay harvest to counter effects of climate change. However, little is known about how this delay occurs. The expression of 1892 genes was significantly changed compared to the control during a 48 h time-course where the auxin 1-Naphthaleneacetic acid (NAA) was applied to pre-veraison grape berries. Principal component analysis showed that the control and auxin-treated samples were most different at 3 h post-treatment when approximately three times more genes were induced than repressed by NAA. There was considerable cross-talk between hormone pathways, particularly between those of auxin and ethylene. Decreased expression of genes encoding putative cell wall catabolic enzymes (including those involved with pectin) and increased expression of putative cellulose synthases indicated that auxins may preserve cell wall structure. This was confirmed by immunochemical labelling of berry sections using antibodies that detect homogalacturonan (LM19) and methyl-esterified homogalacturonan (LM20) and by labelling with the CMB3a cellulose-binding module. Comparison of the auxin-induced changes in gene expression with the pattern of these genes during berry ripening showed that the effect on transcription is a mix of changes that may specifically alter the progress of berry development in a targeted manner and others that could be considered as non-specific changes. Several lines of evidence suggest that cell wall changes and associated berry softening are the first steps in ripening and that delaying cell expansion can delay ripening providing a possible mechanism for the observed auxin effects.
Natural Variation in Adventitious Rooting in the Alpine Perennial Arabis alpina.[Pubmed:32028613]
Plants (Basel). 2020 Feb 3;9(2). pii: plants9020184.
Arctic alpine species follow a mixed clonal-sexual reproductive strategy based on the environmental conditions at flowering. Here, we explored the natural variation for adventitious root formation among genotypes of the alpine perennial Arabis alpina that show differences in flowering habit. We scored the presence of adventitious roots on the hypocotyl, main stem and axillary branches on plants growing in a long-day greenhouse. We also assessed natural variation for adventitious rooting in response to foliar auxin spray. In both experimental approaches, we did not detect a correlation between adventitious rooting and flowering habit. In the greenhouse, and without the application of synthetic auxin, the accession Wca showed higher propensity to produce adventitious roots on the main stem compared to the other accessions. The transcript accumulation of the A. alpina homologue of the auxin inducible GH3.3 gene (AaGH3.3) on stems correlated with the adventitious rooting phenotype of Wca. Synthetic auxin, 1-Naphthaleneacetic acid (1-NAA), enhanced the number of plants with adventitious roots on the main stem and axillary branches. A. alpina plants showed an age-, dosage- and genotype-dependent response to 1-NAA. Among the genotypes tested, the accession Dor was insensitive to auxin and Wca responded to auxin on axillary branches.


