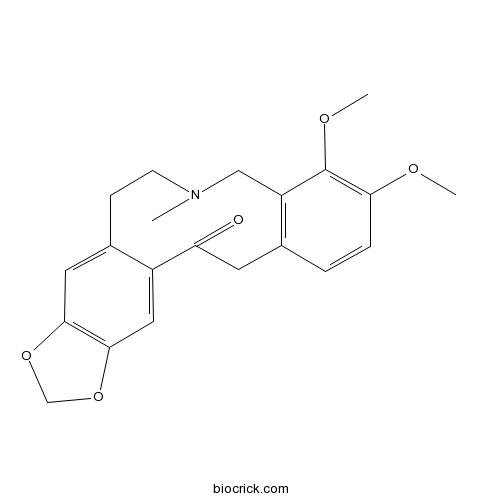
AllocryptopineCAS# 485-91-6 |
- Thalictrimine
Catalog No.:BCN5097
CAS No.:24240-04-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 485-91-6 | SDF | Download SDF |
| PubChem ID | 98570 | Appearance | White powder |
| Formula | C21H23NO5 | M.Wt | 369.42 |
| Type of Compound | Quinolines/Isoquinolines | Storage | Desiccate at -20°C |
| Synonyms | α-Fagarine; β-Homochelidonine | ||
| Solubility | Soluble in chloroform and DMSO | ||
| Chemical Name | 7,8-dimethoxy-11-methyl-17,19-dioxa-11-azatetracyclo[12.7.0.04,9.016,20]henicosa-1(21),4(9),5,7,14,16(20)-hexaen-2-one | ||
| SMILES | CN1CCC2=CC3=C(C=C2C(=O)CC4=C(C1)C(=C(C=C4)OC)OC)OCO3 | ||
| Standard InChIKey | HYBRYAPKQCZIAE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H23NO5/c1-22-7-6-14-9-19-20(27-12-26-19)10-15(14)17(23)8-13-4-5-18(24-2)21(25-3)16(13)11-22/h4-5,9-10H,6-8,11-12H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Allocryptopine Dilution Calculator

Allocryptopine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7069 mL | 13.5347 mL | 27.0695 mL | 54.1389 mL | 67.6737 mL |
| 5 mM | 0.5414 mL | 2.7069 mL | 5.4139 mL | 10.8278 mL | 13.5347 mL |
| 10 mM | 0.2707 mL | 1.3535 mL | 2.7069 mL | 5.4139 mL | 6.7674 mL |
| 50 mM | 0.0541 mL | 0.2707 mL | 0.5414 mL | 1.0828 mL | 1.3535 mL |
| 100 mM | 0.0271 mL | 0.1353 mL | 0.2707 mL | 0.5414 mL | 0.6767 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Secoisolariciresinol
Catalog No.:BCN9042
CAS No.:145265-02-7
- Peimisine hydrochloride
Catalog No.:BCN9041
CAS No.:900498-44-4
- DL-α-Tocopherol
Catalog No.:BCN9040
CAS No.:10191-41-0
- Fluoranthene
Catalog No.:BCN9039
CAS No.:206-44-0
- Ganoderic acid E
Catalog No.:BCN9038
CAS No.:
- D-(+)-Lactose Monohydrate
Catalog No.:BCN9037
CAS No.:64044-51-5
- Kizuta saponin K11
Catalog No.:BCN9036
CAS No.:97240-03-4
- 1-Naphthaleneacetic acid
Catalog No.:BCN9035
CAS No.:86-87-3
- Isopongachromene
Catalog No.:BCN9034
CAS No.:
- Cichoric acid
Catalog No.:BCN9033
CAS No.:6537-80-0
- Anethole, trans-
Catalog No.:BCN9032
CAS No.:4180-23-8
- Galactaric acid
Catalog No.:BCN9031
CAS No.:526-99-8
- Quininic acid
Catalog No.:BCN9044
CAS No.:86-68-0
- Menthol
Catalog No.:BCN9045
CAS No.:89-78-1
- Atractyloside potassium salt
Catalog No.:BCN9046
CAS No.:17754-44-8
- Deslanoside
Catalog No.:BCN9047
CAS No.:17598-65-1
- 1αH,5αH-guaia-6-ene-4β,10β-diol
Catalog No.:BCN9048
CAS No.:2013537-81-8
- Hydroquinone
Catalog No.:BCN9049
CAS No.:123-31-9
- Bisisorhapontigenin D
Catalog No.:BCN9050
CAS No.:
- Mirificin-4'-O-glucoside
Catalog No.:BCN9051
CAS No.:168035-01-6
- DL-Tartaric acid
Catalog No.:BCN9052
CAS No.:133-37-9
- 5'-Cytidylic acid
Catalog No.:BCN9053
CAS No.:63-37-6
- Xanthosine
Catalog No.:BCN9054
CAS No.:146-80-5
- 5'-Guanylic acid
Catalog No.:BCN9055
CAS No.:85-32-5
Natural RNA dependent RNA polymerase inhibitors: Molecular docking studies of some biologically active alkaloids of Argemone mexicana.[Pubmed:32535456]
Med Hypotheses. 2020 Jun 1;144:109905.
COVID-19 has become disastrous for world and spread all over. Researchers all around the globe are working to discover a drug to cure from COVID-19. RNA dependent RNA polymerase plays a key role in SARS-CoV-2 replication and thus it could be a potential target for SARS-CoV-2. This study revealed that Protopine, Allocryptopine and (+/-) 6- Acetonyldihydrochelerythrine could be potential RdRp inhibitors of SARS-CoV-2.
Molecular Network-Guided Alkaloid Profiling of Aerial Parts of Papaver nudicaule L. Using LC-HRMS.[Pubmed:32517053]
Molecules. 2020 Jun 5;25(11). pii: molecules25112636.
Papaver nudicaule L. (Iceland poppy) is widely used for ornamental purposes. A previous study demonstrated the alleviation of lipopolysaccharide-induced inflammation mediated by P. nudicaule extract through nuclear factor-kappa B and signal transducer and activator of transcription 3 inactivation. As isoquinoline alkaloids are chemical markers and bioactive constituents of Papaver species, the present study investigated the alkaloid profile of aerial parts of five P. nudicaule cultivars with different flower colors and a P. rhoeas cropped for two years. A combination of liquid chromatography high-resolution mass spectrometry and molecular networking was used to cluster isoquinoline alkaloids in the species and highlight the possible metabolites. Aside from the 12 compounds, including rotundine, muramine, and Allocryptopine, identified from Global Natural Products Social library and reported information, 46 structurally related metabolites were quantitatively investigated. Forty-two and 16 compounds were proposed for chemical profiles of P. nudicaule and P. rhoeas, respectively. Some species-specific metabolites showed similar fragmentation patterns. The alkaloid abundance of P. nudicaule differed depending on the flower color, and the possible chemical markers were proposed. These results show that molecular networking-guided dereplication allows investigation of unidentified metabolites. The derived chemical profile may facilitate evaluation of P. nudicaule quality for pharmacological applications.
Synthesis and characterization of core-shell magnetic molecularly imprinted polymers for selective extraction of allocryptopine from the wastewater of Macleaya cordata (Willd) R. Br.[Pubmed:32219908]
J Mol Recognit. 2020 Aug;33(8):e2844.
A novel type of magnetic molecularly imprinted polymers (MMIP) as the solid-phase extraction sorbent was prepared, which can extract effectively the Allocryptopine from the waster of Macleaya cordata (Willd) R. Br. In this study, MMIP was synthesized by using Fe3 O4 @SiO2 , 4-vinyl-pyridine, ethylene glycol dimethacrylate, and Allocryptopine, and these ingredients worked as magnetic core, functional monomer, cross-linker, and template, respectively. Concluded by the calculation of Gaussian 09 software, different ratio models of 4-vinyl-pyridine and Allocryptopine were simulated, and the optimal ratio was 1:5 and the energy was -2205.34 kJ/mol. Transmission electron microscopy, vibration sample magnetometry, X-ray diffraction, Fourier transform infrared spectroscopy, and thermogravimetric analysis were used to determine the morphology and structure of MMIP. Furthermore, the results of adsorption experiments indicated that MMIP had high selectivity, excellent recyclability, and good adsorption performance (9.86 mg/g, 298 K). The adsorption process was consistent with the Langmuir adsorption isotherm (R(2) > 0.98, 298 K) and pseudo-second-order kinetics model (R(2) > 0.99, 298 K). After six times adsorption-desorption experiments, the adsorption amount of MMIP only reduced to 8.5%. In the experiments of selective adsorption, MMIP has better adsorption properties for Allocryptopine (ALL, C21 H23 NO5 ) than those having the same functional group. The limit of detection (LOD) was 0.4 mug/mL. The relative standard deviation ranged from 0.09% to 0.72%. The recovery of Allocryptopine in samples ranged from 93.60% to 106.19%. In addition, the synthesized complex had a certain adsorption effect on Allocryptopine separating from the wastewater of Macleaya cordata (Willd) R. Br.
Isolation and purification of alkaloids from the fruits of Macleaya cordata by ionic-liquid-modified high-speed counter-current chromatography.[Pubmed:32175679]
J Sep Sci. 2020 Jun;43(12):2459-2466.
Macleaya cordata (Willd) R. Br. is a medicinal plant. The most important bioactive compounds of M. cordata are alkaloids that have many biological activities including antifungal, anti-inflammatory, and antitumor. In this study, an ionic-liquid-modified high-speed counter-current chromatography method was established to obtain alkaloids from the fruits of M. cordata. The conditions of ionic-liquid-modified high-speed counter-current chromatography, including solvent systems, the content of ionic liquid (1-butyl-3-methylimidazolium tetrafluoroborate [C4 mim][BF4 ]), and the posttreatment of the ionic liquid, were investigated. Five alkaloids protopine, Allocryptopine, sanguinarine, 8-O-demethylchelerythrine, and chelerythrine were separated from the extract of the fruits using a high speed counter-current chromatography with two-phase solvent system composed of dichloromethane/methanol/0.3 mol/L hydrochloric acid aqueous solution/[C4 mim][BF4 ] (4:2:2:0.015, v/v). Their purities were 96.33, 95.56, 97.94, 96.22, and 97.90%, respectively. The results indicated that a small amount of ionic liquids as modifier of the two-phase solvent system could shorten the separation time and improve the separation efficiency of the alkaloids from the fruits. The ionic-liquid-modified high-speed counter-current chromatography would provide a feasible way for highly effective separation of alkaloids from natural products.
Anticancer effects of NSC631570 (Ukrain) in head and neck cancer cells: In vitro analysis of growth, invasion, angiogenesis and gene expression.[Pubmed:31789425]
Oncol Rep. 2020 Jan;43(1):282-295.
NSC631570 (Ukrain) is an aqueous extract of Chelidonium majus, a herbaceous perennial plant, one of two species in the genus Chelidonium, which has been demonstrated to selectively kill tumor cells without affecting nonmalignant cells. In the present study, the components of NSC631570 were examined by combined liquid chromatography/mass spectroscopy (LCMS) and the effects of NSC631570 on HNSCC cell lines, as well as primary cells, were analyzed with respect to growth, apoptosis, invasion, angiogenesis and gene expression. LCMS identified chelerythrine and Allocryptopine as the major alkaloids of the extract. Moreover, NSC631570 suppressed the growth of all tested HNSCC cell lines, including a paclitaxelresistant and Pglycoprotein (MDR1)overexpressing cell line. Mucosal keratinocytes were also affected by the extract, while fibroblasts proved to be much more resistant. In contrast to Allocryptopine, chelerythrine had toxic effects on HNSCC cell lines at low doses. NSC631570 significantly induced apoptosis in the FaDu and HLaC78 cell lines. As analyzed by a spheroidbased invasion assay, cell migration was significantly suppressed by NSC631570 in FaDu cells on gelatine, fibronectin, collagen, laminin and Matrigel(R). Migration of the highly invasive cell line HLaC78 was also inhibited, albeit to a lesser extent (not significant on laminin). Microarray analysis revealed the downregulation of genes encoding key regulators, including EGFR, AKT2, JAK1, STAT3 and sscatenin (CTNNB1), all of which are involved in cell proliferation, migration, angiogenesis, apoptosis as well as the radiation and chemoresistance of HNSCC. The strongest upregulation occurred for cytochrome P450 1A1 (CYP1A1) and 1B1 (CYP1B1), involved in the metabolism of xenobiotics. Upregulation of CYP1A1 was at least partially caused by chelerythrine and Allocryptopine, as shown by RTqPCR in two HNSCC cell lines. In addition, NSC631570 showed a high antiangiogenic action on the tube formation ability of human umbilical vein endothelial cells (HUVECs). In conclusion, this study highlights NSC631570 as a promising therapeutic approach for HNSCC.
Isostructural Solvates of Naturally Occurring Allocryptopine Exhibit Both Mechanochromic and Hydrochromic Luminescent Properties.[Pubmed:31459055]
ACS Omega. 2018 Aug 15;3(8):9220-9226.
We discovered four new solvates for naturally sourced Allocryptopine. The new found crystalline forms are isostructural and have similar crystal structures and packing patterns. However, they exhibit obviously different fluorescence appearance. In addition, the acetone solvate SA, N,N-dimethylformamide solvate SD, and tetrahydrofuran solvate ST also present both luminescent mechanochromic and hydrochromic properties.
Allocryptopine: A Review of Its Properties and Mechanism of Antiarrhythmic Effect.[Pubmed:31389311]
Curr Protein Pept Sci. 2019;20(10):996-1003.
AbstractThroughout the last decade, extensive efforts have been devoted to developing a percutaneous catheter ablation and implantable cardioverter-defibrillator technique for patients suffering from ventricular arrhythmia. Antiarrhythmic drug efficacy for preventing arrhythmias remains disappointing because of adverse cardiovascular effects. Allocryptopine is an isoquinoline alkaloid widely present in medicinal herbs. Studies have indicated that Allocryptopine exhibits potential anti-arrhythmic actions in various animal models. The potential therapeutic benefit of Allocryptopine in arrhythmia diseases is addressed in this study, focusing on multiple ion channel targets and reduced repolarization dispersion. The limitations of Allocryptopine research are clear given a lack of parameters regarding toxicology and pharmacokinetics and clinical efficacy in patients with ventricular arrhythmias. Much remains to be revealed about the properties of Allocryptopine.
Establishment of a rapid and sensitive UPLC-MS/MS method for pharmacokinetic determination of nine alkaloids of crude and processed Corydalis turtschaninovii Besser aqueous extracts in rat plasma.[Pubmed:31233942]
J Chromatogr B Analyt Technol Biomed Life Sci. 2019 Aug 15;1124:218-225.
Corydalis turtschaninovii (CT) is a traditional Chinese medicine which is known to have analgesic effects, and is under investigation for the management of chronic pain. Our study aims to establish a UPLC-MS/MS method for pharmacokinetic determination of nine bioactive alkaloids of raw and processed CT in rat plasma. Nitidine chloride was selected as internal standard. After protein precipitation with methanol, the plasma samples were separated on a reversed phased column with a mobile phase of acetonitrile and water (including 0.1% formic acid). The MRM parameters were optimized as follows: m/z 354.0-->188.1 for protopine, m/z 321.0-->293.1 for coptisine, m/z 371.1-->189.1 for Allocryptopine, m/z 357.1-->193.3 for tetrahydropalmatine, m/z 324.1-->176.1 for tetrahydrocoptisine, m/z 340.1-->176.2 for tetrahydroberberine, m/z 368.1-->289.1 for corynoline, m/z 370.5-->192.1 for corydaline, m/z 367.2-->351.2 for dehydrocorydaline, and m/z 406.0-->300.3 for the IS. The linearity, accuracy, precision, stability, recovery and matrix effect of the method were well validated. This method was successfully employed for a pharmacokinetic study of raw and vinegar-processed CT in rats. The absorption of the nine alkaloids was accomplished in an hour. The double peak phenomenon of the nine alkaloids may be ascribed to enterohepatic recirculation. Compared with the raw group, AUC0-->t and Cmax of the nine alkaloids were significantly elevated in the vinegar-processed group. Our findings suggest that vinegar-processing could increase the bioavailability of the nine alkaloids of CT in rats. The pharmacokinetic information obtained will provide basis for application of processed CT in future clinical therapy.
Change in late sodium current of atrial myocytes in spontaneously hypertensive rats with allocryptopine treatment.[Pubmed:30882133]
Cardiovasc J Afr. 2019 Mar/Apr 23;30(2):79-86.
AIM: We aimed to study the effect of Allocryptopine (All) on the late sodium current (INa,Late) of atrial myocytes in spontaneously hypertensive rats (SHR). METHODS: The enzyme digestion method was used to separate single atrial myocytes from SHR and Wistar-Kyoto (WKY) rats. INa,Late was recorded using the patch-clamp technique, and the effect of All was evaluated on the current. RESULTS: Compared with WKY rat cells, an increase in the INa,Late current in SHR myocytes was found. After treatment with 30 microM All, the current densities were markedly decreased; the ratio of INa,Late/INa,peak of SHR was reduced by 30 microM All. All reduced INa,Late by alleviating inactivation of the channel and increasing the window current of the sodium channel. Furthermore, INa,Late densities of three SCN5A mutations declined substantially with 30 microM All in a concentration-dependent manner. CONCLUSIONS: The results clearly show that an increase in INa,Late in SHR atrial myocytes was inhibited by All derived from Chinese herbal medicine.
TCM-ADMEpred: A novel strategy for poly-pharmacokinetics prediction of traditional Chinese medicine based on single constituent pharmacokinetics, structural similarity, and mathematical modeling.[Pubmed:30826421]
J Ethnopharmacol. 2019 May 23;236:277-287.
ETHNOPHARMACOLOGICAL RELEVANCE: Yuanhu Zhitong prescription (YZP) is a commonly used and relatively simple clinical herb preparation recorded in the China Pharmacopoeia. It contains Corydalis yanhusuo (Chinese name, Yanhusuo [YH]) and Angelica dahurica (Hoffm.) (Chinese name, Baizhi [BZ]), and has a long history of use in traditional Chinese medicine (TCM) for the treatment of stomach pain, hypochondriac pain, headache, and dysmenorrhea. AIM OF THE STUDY: A TCM-ADMEpred method is developed for novel strategy for poly-pharmacokinetics prediction of TCM. To predict the pharmacokinetic characteristics of the main YZP constituents in rat plasma using in silico models, based on the theory that structurally similar constituents show similar pharmacokinetic properties. This approach may facilitate in silico prediction of the pharmacokinetics of TCM. MATERIALS AND METHODS: A robust platform using ultra-performance liquid chromatography coupled with triple quadrupole electrospray tandem mass spectrometry (UPLC-ESI-MS/MS) was developed and validated for simultaneous determination of seven active YZP constituents in rat plasma. These seven compounds were divided into two structural classes, alkaloids and coumarins. The correlation between AUC profiles within a structural class was expressed as Gamma(+), and this variable was used to develop two novel in silico models to predict constituent AUC values. The pharmacokinetics of tetrahydropalmatine, tetrahydroberberine, and corydaline following YZP administration were predicted using the Gamma(+)-values of alpha-Allocryptopine observed following YH administration, while those of imperatorin and isoimperatorin following BZ administration were predicted using the Gamma(+)-values of byakangelicin observed following YZP administration. RESULTS: The UPLC-ESI-MS/MS method was successfully used to evaluate pharmacokinetic parameters after oral YZP, YH, or BZ administration. Our findings showed that co-administration of YH and BZ increased the AUC of four alkaloid constituents and reduced the AUC of three coumarin constituents, which might provide a scientific rationale for co-administering these herbs clinically as a YZP preparation, thus increasing their efficacy and reducing toxicity. The AUC values of imperatorin and isoimperatorin were predicted 3h after oral BZ administration, with the bias ratios between the theoretical values and the observed experimental values ranging from 0.61% to 11.4%, and average bias ratios of 5.8% and 8.0%, respectively. The AUC values of tetrahydropalmatine, tetrahydroberberine, and corydaline were predicted 3h after oral YZP administration, with bias ratios ranging from 3.7% to 46.4%, and average bias ratios of 23.8%, 15.4%, and 25.8%, respectively. CONCLUSION: The UPLC-ESI-MS/MS method was successfully applied to pharmacokinetic evaluations after oral administration of YZP, YH, and BZ to rats. The Gamma(+) variable was used to express the correlation between the AUC profiles of structurally similar compounds. This facilitated the development of an in silico model that was used to predict the AUC of three alkaloids in YZP and of two coumarins in BZ. Calculation of the bias ratios between the predicted and experimental values suggested that this in silico model provided a viable approach for the prediction of TCM pharmacokinetics.
Profiling and Pharmacokinetic Studies of Alkaloids in Rats After Oral Administration of Zanthoxylum nitidum Decoction by UPLC-Q-TOF-MS/MS and HPLC-MS/MS.[Pubmed:30736390]
Molecules. 2019 Feb 7;24(3). pii: molecules24030585.
Zanthoxylum nitidum (Roxb.) DC (Rutaceae), called as "liangmianzhen" in China, is well known for its anti-inflammation and analgesic effect. Alkaloids are its main active constituents. However, little has been known about the absorption of main alkaloids in vivo. In this study, an ultra-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry was employed for identification of absorbed alkaloids in rats after oral administration of Z. nitidum decoction. By analyzing the fragmentation patterns, a total of nineteen alkaloids were exactly or tentatively identified in rat plasma after treatment, of which magnoflorine, alpha-Allocryptopine, and skimmianine are dominant. Moreover, a high performance liquid chromatography coupled mass spectrometry method was developed for simultaneous quantification of magnoflorine, alpha-Allocryptopine, and skimmianine, and successfully applied to pharmacokinetic study in rats after oral administration of Z. nitidum decoction. The research would contribute to comprehensive understanding of the material basis and function mechanism of Z. nitidum decoction.
Sensing of Different Human Telomeric G-Quadruplex DNA Topologies by Natural Alkaloid Allocryptopine Using Spectroscopic Techniques.[Pubmed:30346761]
J Phys Chem B. 2018 Nov 15;122(45):10279-10290.
This article describes how a natural alkaloid Allocryptopine (ALL) is able to differentiate two forms of biologically relevant human telomeric (htel22) G-quadruplex DNAs (GQ-DNA) depending on the presence of K(+) and Na(+) ions by steady-state and time-resolved spectroscopic techniques. For both interactions, predominant involvements of static-type quenching mechanism with the negligible influence of dynamic collision are established by UV-vis absorption and fluorescence emission study, which is further supported by fluorescence lifetime measurements. ALL exhibits appreciable affinity toward both GQ-DNAs. Both the mixed-hybrid (3 + 1) quadruplex structures in K(+) ions and the basket-type antiparallel quadruplex structure under Na(+) condition are converted to parallel types in the presence of ALL. Fluorescence intercalator displacement assay experiment revealed modest selectivity of ALL to both quadruplexes over duplex DNA along with higher selectivity for antiparallel types among the two quadruplexes via groove and/or loop binding, which is distinct from the conventional pi-stacking of the ligands on external G-quartets. ALL stabilized both GQ-DNA topologies moderately. The differences in the dynamics of ALL within both DNA environments have been demonstrated vividly by time-resolved anisotropy measurements using the wobbling-in-cone model. These results suggest groove binding with antiparallel G-quartet with high affinity and moderate loop binding with mixed-hybrid G-quartet accompanied by the partial end stacking additionally in both of the cases. Our conclusions are further supported by steady-state anisotropy measurements and molecular docking. The present investigation can be used in the development of a biocompatible antitumour/anticancer agent targeting particular GQ-DNA conformation.
Identification of quality markers of Yuanhu Zhitong tablets based on integrative pharmacology and data mining.[Pubmed:29551644]
Phytomedicine. 2018 May 15;44:212-219.
BACKGROUND: The quality evaluation of traditional Chinese medicine (TCM) formulations is needed to guarantee the safety and efficacy. In our laboratory, we established interaction rules between chemical quality control and biological activity evaluations to study Yuanhu Zhitong tablets (YZTs). Moreover, a quality marker (Q-marker) has recently been proposed as a new concept in the quality control of TCM. However, no appropriate methods are available for the identification of Q-markers from the complex TCM systems. PURPOSE: We aimed to use an integrative pharmacological (IP) approach to further identify Q-markers from YZTs through the integration of multidisciplinary knowledge. In addition, data mining was used to determine the correlation between multiple constituents of this TCM and its bioactivity to improve quality control. METHODS: The IP approach was used to identify the active constituents of YZTs and elucidate the molecular mechanisms by integrating chemical and biosynthetic analyses, drug metabolism, and network pharmacology. Data mining methods including grey relational analysis (GRA) and least squares support vector machine (LS-SVM) regression techniques, were used to establish the correlations among the constituents and efficacy, and dose efficacy in multiple dimensions. RESULTS: Seven constituents (tetrahydropalmatine, alpha-Allocryptopine, protopine, corydaline, imperatorin, isoimperatorin, and byakangelicin) were identified as Q-markers of YZT using IP based on their high abundance, specific presence in the individual herbal constituents and the product, appropriate drug-like properties, and critical contribution to the bioactivity of the mixture of YZT constituents. Moreover, three Q-markers (protopine, alpha-Allocryptopine, and corydaline) were highly correlated with the multiple bioactivities of the YZTs, as found using data mining. Finally, three constituents (tetrahydropalmatine, corydaline, and imperatorin) were chosen as minimum combinations that both distinguished the authentic components from false products and indicated the intensity of bioactivity to improve the quality control of YZTs. CONCLUSIONS: Tetrahydropalmatine, imperatorin, and corydaline could be used as minimum combinations to effectively control the quality of YZTs.


