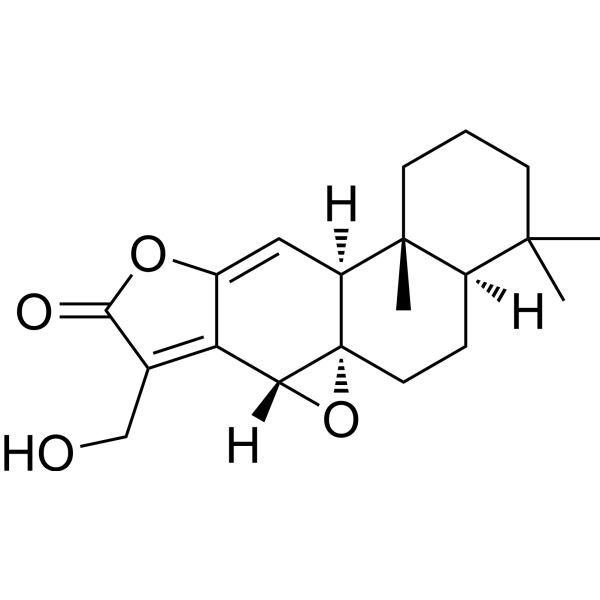
17-Hydroxyjolkinolide ACAS# 65388-16-1 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 65388-16-1 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C20H26O4 | M.Wt | 330.42 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Caudicifolin,Fischeriana A | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

17-Hydroxyjolkinolide A Dilution Calculator

17-Hydroxyjolkinolide A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0265 mL | 15.1323 mL | 30.2645 mL | 60.529 mL | 75.6613 mL |
| 5 mM | 0.6053 mL | 3.0265 mL | 6.0529 mL | 12.1058 mL | 15.1323 mL |
| 10 mM | 0.3026 mL | 1.5132 mL | 3.0265 mL | 6.0529 mL | 7.5661 mL |
| 50 mM | 0.0605 mL | 0.3026 mL | 0.6053 mL | 1.2106 mL | 1.5132 mL |
| 100 mM | 0.0303 mL | 0.1513 mL | 0.3026 mL | 0.6053 mL | 0.7566 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2,2′-Dihydroxy-4,6-dimethoxy-3-methylacetophenone
Catalog No.:BCX1865
CAS No.:184706-61-4
- Heilaohuguosu F
Catalog No.:BCX1864
CAS No.:2763686-96-8
- Heteroclitin A
Catalog No.:BCX1863
CAS No.:140369-75-1
- Spinasaponin E
Catalog No.:BCX1862
CAS No.:2867584-02-7
- β-Patchoulene
Catalog No.:BCX1861
CAS No.:514-51-2
- (-)-Bornyl ferulate
Catalog No.:BCX1860
CAS No.:55511-07-4
- Isovaleroyl oxokadsuranol
Catalog No.:BCX1859
CAS No.:130252-45-8
- Renchangianin B
Catalog No.:BCX1858
CAS No.:770733-54-5
- Kadsuphilin J
Catalog No.:BCX1857
CAS No.:1017242-94-2
- Kadsulignan C
Catalog No.:BCX1856
CAS No.:137637-49-1
- Stauntoside M
Catalog No.:BCX1855
CAS No.:1537167-62-6
- Interiotherin D
Catalog No.:BCX1854
CAS No.:460090-66-8
- Euphoscopin B
Catalog No.:BCX1867
CAS No.:81557-52-0
- Epieuphoscopin B
Catalog No.:BCX1868
CAS No.:126372-45-0
- Marstenacisside F1
Catalog No.:BCX1869
CAS No.:2902666-68-4
- Marsdenoside A
Catalog No.:BCX1870
CAS No.:858360-56-2
- Machilin A
Catalog No.:BCX1871
CAS No.:110269-50-6
- Glomeratide A
Catalog No.:BCX1872
CAS No.:1072028-74-0
- Tenuiphenone B
Catalog No.:BCX1873
CAS No.:870298-00-3
- Strictinin
Catalog No.:BCX1874
CAS No.:517-46-4
- Euphorblin R
Catalog No.:BCX1875
CAS No.:2230806-06-9
- Ingol-7,8,12-triacetate-3-phenylacetate
Catalog No.:BCX1876
CAS No.:944799-46-6
- 11α-O-Tigloyl-12β-O-acetyltenacigenin B
Catalog No.:BCX1877
CAS No.:154022-51-2
- Marsdenoside B
Catalog No.:BCX1878
CAS No.:858360-57-3
Chemical Composition, Anti-Breast Cancer Activity and Extraction Techniques of Ent-Abietane Diterpenoids from Euphorbia fischeriana Steud.[Pubmed:35807527]
Molecules. 2022 Jul 3;27(13):4282.
Ent-abietane diterpenoids are the main active constituents of Euphorbia fischeriana. In the continuing search for new anti-breast cancer drugs, 11 ent-abietane diterpenoids (1-11) were isolated from E. fischeriana. The structures of these compounds were clearly elucidated on the basis of 1D and 2D NMR spectra as well as HRESIMS data. Among them, compound 1 was a novel compound, compound 10 was isolated from Euphorbia genus for the first time, compound 11 was firstly discovered from E. fischeriana. These compounds exhibited varying degrees of growth inhibition against the MCF-10A, MCF-7, ZR-75-1 and MDA-MB-231 cell lines in vitro. The experimental data obtained permit us to identify the roles of the epoxy group, hydroxyl group and acetoxyl group on their cytotoxic activities. Extraction is an important means for the isolation, identification, and application of valuable compounds from natural plants. To maximize yields of ent-abietane diterpenoids of E. fischeriana, 17-hydroxyjolkinolide B, jolkinolide B, 17-Hydroxyjolkinolide A and jolkinolide A were selected as quality controls to optimize the salting-out-assisted liquid-liquid extraction (SALLE) by response surface methodology (RSM). The optimized conditions for SALLE were 0.47 g sodium dihydrogen phosphate, 5.5 mL acetonitrile and 4.5 mL water at pH 7.5. The experimental values of 17-hydroxyjolkinolide B, jolkinolide B, 17-Hydroxyjolkinolide A and jolkinolide A (2.134, 0.529, 0.396, and 0.148 mg/g, respectively) were in agreement with the predicted values, thus demonstrating the appropriateness of the model.
Optimization of extraction for diterpenoids from Euphorbia fischeriana Steud using response surface methodology and structure identification by UPLC-Q-TOF-MS.[Pubmed:31621425]
Nat Prod Res. 2021 Jul;35(14):2458-2462.
Jolkinolide A, jolkinolide B, 17-Hydroxyjolkinolide A and 17-hydroxyjolkinolide B are abundant constitutes in Euphorbia Fischeriana Steud and exhibit profound bioactivities. In this study, they were selected as quality control to optimize the extraction of E. fischeriana. Response surface methodology employing Box-Behnken design was applied to test the optimal conditions for the extraction. The optimized conditions for the simultaneous extraction of four diterpenoids from E. fischeriana were: ethanol concentration 100%, extraction temperature 74 degrees C and extraction time 2.0 h. The extraction contents for jolkinolide A, jolkinolide B, 17-Hydroxyjolkinolide A and 17-hydroxyjolkinolide B were 0.1763, 0.9643, 0.4245 and 2.8189 mg/g. The extract obtained under the optimal conditions was injected into UPLC-Q-TOF-MS system. Fifty-one peaks were identified. Two peaks were tentatively identified as new compounds. The compounds were diterpenoids, fatty oil, phenolics and others.
Feeding deterrents against two grain storage insects from Euphorbia fischeriana.[Pubmed:21221063]
Molecules. 2011 Jan 10;16(1):466-76.
The screening of several Chinese medicinal herbs for insecticidal principles showed that Euphorbia fischeriana roots possessed significant feeding deterrent activity against two stored-product insects (Tribolium castaneum and Sitophilus zeamais). From ethanol extract, four feeding deterrents were isolated by bioassay-guided fractionation. The compounds were identified as jolkinolide B, 12-deoxyphorbol 13-(9Z)-octadecenoate 20-acetate, 17-Hydroxyjolkinolide A and B on the basis of their phytochemical and spectral data. Jolkinolide B and 17-hydroxyjolkinolide B possessed strong feeding deterrent activities against S. zeamais (EC(5)(0) = 342.1 and 543.9 ppm, respectively) and T. castaneum adults (E(5)(0) = 361.4 and 551.5 ppm, respectively). 17-Hydroxyjolkinolide A and 12-deoxyphorbol 13-(9Z)-octadecenoate 20-acetate A also exhibited feeding deterrent activity against the two grain storage insects with EC(5)(0) values of 631.9 and 884.3 ppm for S. zeamais and 656.5 and 1058.4 ppm for T. castaneum adults.


