Machilin ACAS# 110269-50-6 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 110269-50-6 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
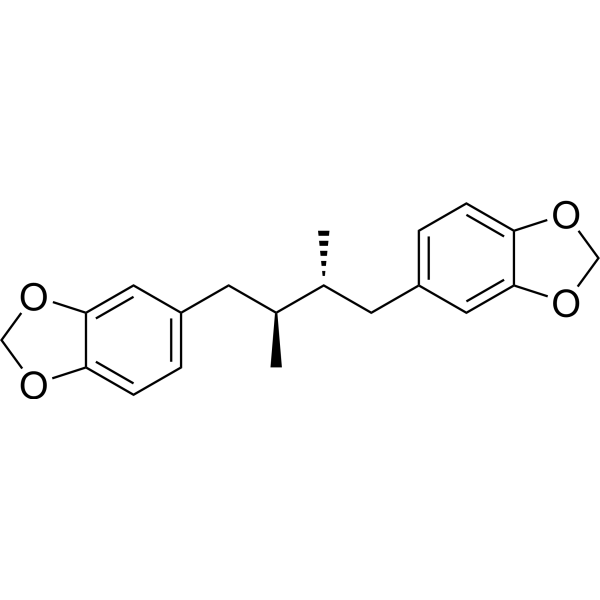
| Formula | C20H22O4 | M.Wt | 326.39 |
| Type of Compound | Lignanoids | Storage | Desiccate at -20°C |
| Synonyms | erythro-Austrobailignan-5,meso-Austrobailignan-5 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Machilin A Dilution Calculator

Machilin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0638 mL | 15.3191 mL | 30.6382 mL | 61.2764 mL | 76.5955 mL |
| 5 mM | 0.6128 mL | 3.0638 mL | 6.1276 mL | 12.2553 mL | 15.3191 mL |
| 10 mM | 0.3064 mL | 1.5319 mL | 3.0638 mL | 6.1276 mL | 7.6595 mL |
| 50 mM | 0.0613 mL | 0.3064 mL | 0.6128 mL | 1.2255 mL | 1.5319 mL |
| 100 mM | 0.0306 mL | 0.1532 mL | 0.3064 mL | 0.6128 mL | 0.766 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Marsdenoside A
Catalog No.:BCX1870
CAS No.:858360-56-2
- Marstenacisside F1
Catalog No.:BCX1869
CAS No.:2902666-68-4
- Epieuphoscopin B
Catalog No.:BCX1868
CAS No.:126372-45-0
- Euphoscopin B
Catalog No.:BCX1867
CAS No.:81557-52-0
- 17-Hydroxyjolkinolide A
Catalog No.:BCX1866
CAS No.:65388-16-1
- 2,2′-Dihydroxy-4,6-dimethoxy-3-methylacetophenone
Catalog No.:BCX1865
CAS No.:184706-61-4
- Heilaohuguosu F
Catalog No.:BCX1864
CAS No.:2763686-96-8
- Heteroclitin A
Catalog No.:BCX1863
CAS No.:140369-75-1
- Spinasaponin E
Catalog No.:BCX1862
CAS No.:2867584-02-7
- β-Patchoulene
Catalog No.:BCX1861
CAS No.:514-51-2
- (-)-Bornyl ferulate
Catalog No.:BCX1860
CAS No.:55511-07-4
- Isovaleroyl oxokadsuranol
Catalog No.:BCX1859
CAS No.:130252-45-8
- Glomeratide A
Catalog No.:BCX1872
CAS No.:1072028-74-0
- Tenuiphenone B
Catalog No.:BCX1873
CAS No.:870298-00-3
- Strictinin
Catalog No.:BCX1874
CAS No.:517-46-4
- Euphorblin R
Catalog No.:BCX1875
CAS No.:2230806-06-9
- Ingol-7,8,12-triacetate-3-phenylacetate
Catalog No.:BCX1876
CAS No.:944799-46-6
- 11α-O-Tigloyl-12β-O-acetyltenacigenin B
Catalog No.:BCX1877
CAS No.:154022-51-2
- Marsdenoside B
Catalog No.:BCX1878
CAS No.:858360-57-3
- 3-Epidigitoxigenin
Catalog No.:BCX1879
CAS No.:545-52-8
- Chrysindin A
Catalog No.:BCX1880
CAS No.:1374852-85-3
- Pebrellin
Catalog No.:BCX1881
CAS No.:13509-93-8
- Hymenoxin
Catalog No.:BCX1882
CAS No.:56003-01-1
- Dehydrodanshenol A
Catalog No.:BCX1883
CAS No.:1444618-61-4
Lignans from Machilus thunbergii as Thymic Stromal Lymphopoietin Inhibitors.[Pubmed:34443392]
Molecules. 2021 Aug 8;26(16):4804.
Thymic stromal lymphopoietin (TSLP) plays an important role in the pathophysiology of various allergic diseases that are mediated by T helper cell type-2 (Th2) responses, including asthma and atopic dermatitis. The primary focus of this study was the identification of potent inhibitors of the TSLP signaling pathway for potential therapeutic use. The 80% methanol extract of Machilus thunbergii bark significantly inhibited the signal transducer and activator of transcription 5 (STAT5) phosphorylation in human mast cell (HMC)-1 cells. Through activity-guided isolation, three lignans (1-3) were obtained and identified as (+)-galbelgin (1), meso-dihydroguaiaretic acid (2), and Machilin A (3). Among them, two lignans (1 and 2) significantly inhibited STAT5 phosphorylation and TSLP/TSLPR interaction, as determined by ELISA. Our results indicated that lignans isolated from M. thunbergii are a promising resource for the treatment of allergic diseases.
Machilin A Inhibits Tumor Growth and Macrophage M2 Polarization Through the Reduction of Lactic Acid.[Pubmed:31324019]
Cancers (Basel). 2019 Jul 9;11(7):963.
Lactate dehydrogenase A (LDHA) is an important enzyme responsible for cancer growth and energy metabolism in various cancers via the aerobic glycolytic pathway. Here, we report that Machilin A (MA), which acts as a competitive inhibitor by blocking the nicotinamide adenine dinucleotide (NAD) binding site of LDHA, suppresses growth of cancer cells and lactate production in various cancer cell types, including colon, breast, lung, and liver cancers. Furthermore, MA markedly decreased LDHA activity, lactate production, and intracellular adenosine triphosphate (ATP) levels induced by hypoxia-induced LDHA expression in cancer cells, and significantly inhibited colony formation, leading to reduced cancer cell survival. In mouse models inoculated with murine Lewis lung carcinoma, MA significantly suppressed tumor growth as observed by a reduction of tumor volume and weight; resulting from the inhibition of LDHA activity. Subsequently, the suppression of tumor-derived lactic acid in MA-treated cancer cells resulted in decrease of neovascularization through the regulation of alternatively activated macrophages (M2) polarization in macrophages. Taken together, we suggest that the reduction of lactate by MA in cancer cells directly results in a suppression of cancer cell growth. Furthermore, macrophage polarization and activation of endothelial cells for angiogenesis were indirectly regulated preventing lactate production in MA-treated cancer cells.
Selective inhibitory effects of machilin A isolated from Machilus thunbergii on human cytochrome P450 1A and 2B6.[Pubmed:26055126]
Phytomedicine. 2015 Jun 1;22(6):615-20.
BACKGROUND: The bark of Machilus thunbergii (Lauraceae) has been used as a folk medicine to treat abdominal pain and distension, and leg edema in Korea. Machilin A (MA), a lignan isolated from Machilus thunbergii, exhibits several biological activities including anti-oxidant and stimulatory effects on cell differentiation and proliferation. PURPOSE: Potential drug-interactions with MA via inhibition of cytochrome P450 (CYP) activity in human liver microsomes (HLMs), have not been investigated. STUDY DESIGN: The inhibitory effects of MA on the activities of CYPs were investigated using cocktail probe substrates in pooled HLMs and on human recombinant cDNA-expressed CYP isoforms. METHODS: The nine CYP-specific substrates were incubated in HLM or recombinant cDNA-expressed CYP 1A1, 1A2 and 2B6 with MA. After incubation, the samples were injected onto a C18 column for liquid chromatography-tandem mass spectrometry analysis. To investigate the binding poses between MA and CYP, we carried out structure-based docking simulations by using software and scripts written in-house (ALIS-DOCK; Automatic pLatform for Iterative Structure-based DOCKing). RESULTS: MA strongly inhibited CYP1A2-mediated phenacetin O-deethylation and CYP2B6-mediated bupropion hydroxylation with IC50 values of 3.0 and 3.9 microM, respectively, while it did not significantly inhibit other CYPs. A Dixon plot indicated that MA competitively inhibits CYP1A2 and CYP2B6 with Ki values of 0.71 and 4.1 microM, respectively. CONCLUSION: Overall, this was the first investigation of the inhibitory effects of MA on CYP1A2 and CYP2B6 in HLMs, and it has identified that MA acts via competitive inhibition.
Dibenzylbutane lignans from the stems of Schisandra bicolor.[Pubmed:24079183]
Nat Prod Commun. 2013 Aug;8(8):1121-2.
Further investigation of the stems of Schisandra bicolor led to the isolation of a new dibenzylbutane lignan, named schibicolignan A (1), as well as five known compounds, namely bis[dibenzylbutane] (2), Machilin A (3), macelignan (4), saururenin (5) and sphenanlignan (6). The structure of the new lignan was elucidated on the basis of extensive spectroscopic analysis. Antioxidant activity of 1-6 was also evaluated.
Machilin A isolated from Myristica fragrans stimulates osteoblast differentiation.[Pubmed:19096999]
Planta Med. 2009 Feb;75(2):152-7.
This study evaluated the stimulatory effects of Machilin A and structurally related lignans isolated from Myristica fragrans on osteoblast differentiation. In two IN VITRO osteoblast differentiation models, Machilin A stimulated osteoblast differentiation via activation of p38 MAP kinase. Lignans isolated from Myristica fragrans also stimulated osteoblast differentiation in MC3T3-E1 cells; the lignans included macelignan, machilin F, nectandrin B, safrole, licarin A, licarin B, myristargenol, and meso-dihydroguaiaretic acid. These data suggest that lignans isolated from Myristica fragrans have anabolic activity in bone metabolism.
Stimulatory activity of lignans from Machilus thunbergii on osteoblast differentiation.[Pubmed:17409528]
Biol Pharm Bull. 2007 Apr;30(4):814-7.
Phytoestrogens are naturally occurring compounds exerting estrogenic activity, and include isoflavonoids, flavonoids and lignans. In the present study, we evaluated the stimulating activity of six lignans, meso-dihydroguaiaretic acid, nordihydroguaiaretic acid, Machilin A, guaiacin, isoguaiacin and isoguaiacin dimethylether, from Machilus thunbergii, on osteoblast differentiation employing primary cultures of mouse osteoblast as an in vitro assay system. Among the six lignans tested, arylnaphthalene type lignans such as guaiacin, isoguaiacin and isoguaiacin dimethylether significantly increased alkaline phosphatase activity, whereas bibenzylbutane type lignans such as meso-dihydroguaiaretic acid, nordihydroguaiaretic acid and Machilin A showed little effects. Isoguaiacin and isoguaiacin dimethylether also increased collagen synthesis as well as calcium deposition. In addition, treatment of the mouse osteoblasts with tamoxifen markedly reduced ALP activity increased by isoguaiacin or isoguaiacin dimethylether, suggesting the involvement of estrogen receptor in the action of these lignans on osteoblast differentiation. Taken together, these results suggest that arylnaphthalene type lignans such as guaiacin, isoguaiacin and isoguaiacin dimethylether significantly increase osteoblast differentiation.
Inhibition of phospholipase Cgamma1 and cancer cell proliferation by lignans and flavans from Machilus thunbergii.[Pubmed:15554262]
Arch Pharm Res. 2004 Oct;27(10):1043-7.
Thirteen compounds were isolated from the CH2Cl2 fraction of Machilus thunbergii as phospholipase Cgamma1 (PLCgamma1) inhibitors. These compounds were identified as nine lignans, two neolignans, and two flavans by spectroscopic analysis. Of these, 5,7-di-O-methyl-3',4'-methylenated (-)-epicatechin (12) and 5,7,3'-tri-O-methyl (-)-epicatechin (13) have not been reported previously in this plant. In addition, seven compounds, Machilin A (1), (-)-sesamin (3), machilin G (5), (+)-galbacin (9), licarin A (10), (-)-acuminatin (11) and compound 12 showed dose-dependent potent inhibitory activities against PLCgamma1 in vitro with IC50 values ranging from 8.8 to 26.0 microM. These lignans, neolignans, and flavans are presented as a new class of PLCgamma1 inhibitors. The brief study of the structure activity relationship of these compounds suggested that the benzene ring with the methylene dioxy group is responsible for the expression of inhibitory activities against PLCgamma1. Moreover, it is suggested that inhibition of PLCgamma1 may be an important mechanism for an antiproliferative effect on the human cancer cells. Therefore, these inhibitors may be utilized as cancer chemotherapeutic and chemopreventive agents.
Increase of caspase-3 activity by lignans from Machilus thunbergii in HL-60 cells.[Pubmed:15305043]
Biol Pharm Bull. 2004 Aug;27(8):1305-7.
Nine lignans and two butanolides were isolated from the stem bark of Machilus thunbergii and their structures were identified as Machilin A (1), licarin B (2), zuonin B (3), macelignan (4), secoisolancifolide (5), isolancifolide (6), oleiferin C (7), meso-dihydroguaiaretic acid (8), licarin A (9), machilin F (10), and nectandrin B (11) by spectroscopic means. These compounds were assessed for their abilities to activate a caspase-3 activity in human promyeloid leukemic HL-60 cells. The intracellular caspase-3 activity of macelignan (4), oleiferin C (7), meso-dihydroguaiaretic acid (8), and licarin A (9) increased approximately 3.04, 6.16, 2.10, and 3.10-fold at 100 microM over that of untreated control. In addition, compounds 4, 7, 8, and 9 induced internucleosomal DNA fragmentation in HL-60 cells.
Lignans from the bark of Machilus thunbergii and their DNA topoisomerases I and II inhibition and cytotoxicity.[Pubmed:15256759]
Biol Pharm Bull. 2004 Jul;27(7):1147-50.
Activity-guided fractionation based on topoisomerase I inhibitory activity lead to the isolation of ten lignans (1-10) from the methylene chloride extract of the bark of Machilus thunbergii SIEB. et ZUCC. (Lauraceae). These were identified as Machilin A (1), erythro-austrobailignan-6 (2), meso-monomethyl dihydroguaiaretic acid (3), meso-dihydroguaiaretic acid (4), galbacin (5), machilin F (6), nectandrin A (7) nectandrin B (8), (-)-acuminatin (9) and (7S,8S)-7-(4-hydroxy-3-methoxyphenyl)-1'-formyl-3'-methoxy-8-methyldihydrobenzofuran (10) by spectral evidence. In DNA topoisomerase I and II assays in vitro at a concentration of 100 microM, 4 showed the most potent inhibitory activity, 93.6 and 82.1% inhibition, respectively, and 8 showed 79.1 and 34.3% inhibition, respectively. All of these compounds exhibited weak or no cytotoxicities against either the human colon carcinoma cell line (HT-29) or the human breast carcinoma cell line (MCF-7).
Neuroprotective lignans from the bark of Machilus thunbergii.[Pubmed:14765301]
Planta Med. 2004 Jan;70(1):79-80.
The CH (2)Cl (2) fraction of the bark of Machilus thunbergii Sieb. et Zucc. (Lauraceae) significantly protected primary cultures of rat cortical cells exposed to the excitotoxic amino acid, L-glutamate. (-)-Isoguaiacin, meso-dihydroguaiaretic acid, Machilin A, (+)-galbelgin, licarin A, (-)-sesamin, and (+)-guaiacin were isolated by bioactivity-guided fractionation from the CH (2)Cl (2) fraction and further separated using chromatographic techniques. Isoguaiacin, meso-dihydroguaiaretic acid, licarin A and (+)-guaiacin had significant neuroprotective activities against glutamate-induced neurotoxicity in primary cultures of rat cortical cells at concentrations ranging from 0.1 microM to 10.0 microM.
Melanin biosynthesis inhibitors from the bark of Machilus thunbergii.[Pubmed:12843636]
Biol Pharm Bull. 2003 Jul;26(7):1039-41.
The bioassay-guided fractionation of the methylene chloride soluble portion of a methanol extract of Machilus thunbergii bark led to the isolation of four known lignans, Machilin A (1), meso-monomethyl dihydroguaiaretic acid (2), nectandrin A (3) and nectandrin B (4), which exhibited potent inhibitory activity on melanin biosynthesis in cultured B-16 mouse melanoma cells (IC(50): 39.9, 15.1, 19.4 and 37.8 microM, respectively).
Antioxidant lignans from Machilus thunbergii protect CCl4-injured primary cultures of rat hepatocytes.[Pubmed:11045899]
J Pharm Pharmacol. 2000 Sep;52(9):1163-9.
Eleven lignans (1-11) were isolated from the CH2Cl2 fraction of the bark of Machilus thunbergii Sieb. et Zucc. (Lauraceae). These were identified as (-)-acuminatin (1), (-)-isoguaiacin (2), meso-dihydroguaiaretic acid (3), (+)-galbacin (4), (-)-sesamin (5), (+)-galbelgin (6), Machilin A (7), machilin G (8), licarin A (9), and nectandrin A (10) and B (11). Primary cultures of rat hepatocytes were co-incubated for 90 min with the hepatotoxin CCl4 and each of the 11 lignans (50 microM). Hepatoprotective activity was determined by measuring the level of glutamic pyruvic transaminase released into the medium from the primary cultures of rat hepatocytes. (-)-Acuminatin, (-)-isoguaiacin and meso-dihydroguaiaretic acid all significantly reduced the level of glutamic pyruvic transaminase released. Further investigation revealed that these three compounds significantly preserved the levels and the activities of glutathione, superoxide dismutase, glutathione peroxidase and catalase. (-)-Acuminatin, (-)-isoguaiacin and meso-dihydroguaiaretic acid also ameliorated lipid peroxidation as demonstrated by a reduction of malondialdehyde production. These results suggest that (-)-acuminatin, (-)-isoguaiacin and meso-dihydroguaiaretic acid exert diverse hepatoprotective activities, perhaps by serving as potent antioxidants.
Natural flavonoids and lignans are potent cytostatic agents against human leukemic HL-60 cells.[Pubmed:8084211]
Life Sci. 1994;55(13):1061-9.
Anti leukemic-cell efficacy of 28 naturally occurring and synthetic flavonoids and 11 naturally occurring lignans on human promyelocytic leukemic cell line HL-60 were examined using MTT assay methods. Differences between anti cell-proliferative activity and cytotoxicity of these compounds were compared with those of 4 clinical anti-cancer agents. Eight of the 28 flavonoids and 4 of the 11 lignans showed considerable suppressive effects on HL-60 cell growth with IC50s ranging from 10-940 ng/ml. Among these compounds, genistein, honokiol, Machilin A, matairesinol, and arctigenin had the strongest effects with IC50s less than 100 ng/ml, which were almost equivalent to the effects of current anti-cancer agents. The flavonoid genistein and the lignans, however, showed little or no cytotoxicity against HL-60 cells as assessed by dye exclusion tests (LC50s > 2,900 ng/ml), whereas the regular anti-cancer agents had potent cytotoxicity. All of the flavonoids and lignans, except for Machilin A and arctigenin, were less effective against growth of human T lymphocytic leukemia cell line MOLT-4. In addition, the flavonoid and the lignans showed little or no inhibiting activity on mitogen-induced blastogenesis of human peripheral-blood lymphocytes. The lignans and genistein were strongly suppressive against incorporations of [3H]thymidine, [3H]uridine, and [3H]leucine into HL-60 cells. These results showed that some of the naturally occurring flavonoids and lignans inhibited HL-60 cell growth with a non-toxic mechanism, possibly via cessation of DNA, RNA, and/or protein synthesis of the leukemic cells.
Suppression of mitogen-induced proliferation of human peripheral blood lymphocytes by plant lignans.[Pubmed:1663632]
Planta Med. 1991 Aug;57(4):331-4.
The effects of seven lignans and two neolignans derived from plants and herbs on the concanavalin A-induced proliferation of human peripheral blood lymphocytes in vitro were studied. All compounds showed inhibitory activity with an IC50, ranging from 0.02 to 4.30 micrograms/ml (1.6 x 10(-8) to 1.6 x 10(-5) M). Machilin A (2,3-dimethyl-1,4-dipiperonylbutane), a Lauraceae lignan, was the strongest inhibitor and was quite effective as the synthetic immunosuppressive glucocorticoid prednisolone. The viability of lymphocytes before and after treatment, as assessed by a dye exclusion test, indicated no change, and thus the lignans are not lymphocytotoxic but may inhibit DNA synthesis. The results suggest the value of further assessment of plant lignans as immunosuppressive agents.


