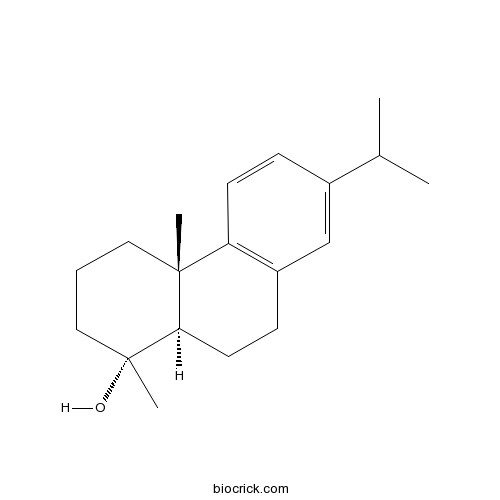
18-Norabieta-8,11,13-trien-4-olCAS# 22478-65-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 22478-65-5 | SDF | Download SDF |
| PubChem ID | 15605917 | Appearance | Powder |
| Formula | C19H28O | M.Wt | 272.4 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,4aS,10aR)-1,4a-dimethyl-7-propan-2-yl-2,3,4,9,10,10a-hexahydrophenanthren-1-ol | ||
| SMILES | CC(C)C1=CC2=C(C=C1)C3(CCCC(C3CC2)(C)O)C | ||
| Standard InChIKey | SOJWLJKPIIODOH-GUDVDZBRSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

18-Norabieta-8,11,13-trien-4-ol Dilution Calculator

18-Norabieta-8,11,13-trien-4-ol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6711 mL | 18.3554 mL | 36.7107 mL | 73.4214 mL | 91.7768 mL |
| 5 mM | 0.7342 mL | 3.6711 mL | 7.3421 mL | 14.6843 mL | 18.3554 mL |
| 10 mM | 0.3671 mL | 1.8355 mL | 3.6711 mL | 7.3421 mL | 9.1777 mL |
| 50 mM | 0.0734 mL | 0.3671 mL | 0.7342 mL | 1.4684 mL | 1.8355 mL |
| 100 mM | 0.0367 mL | 0.1836 mL | 0.3671 mL | 0.7342 mL | 0.9178 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gymnemagenin
Catalog No.:BCN7841
CAS No.:22467-07-8
- MLCK inhibitor peptide 18
Catalog No.:BCC5828
CAS No.:224579-74-2
- Benfotiamine
Catalog No.:BCC1415
CAS No.:22457-89-2
- Retapamulin
Catalog No.:BCC4837
CAS No.:224452-66-8
- Ginsenoside Rg1
Catalog No.:BCN1066
CAS No.:22427-39-0
- Bayogenin methyl ester
Catalog No.:BCN3722
CAS No.:22425-81-6
- 2,2'-Anhydro-5-methyluridine
Catalog No.:BCC8486
CAS No.:22423-26-3
- Incensole
Catalog No.:BCN3831
CAS No.:22419-74-5
- Siramesine hydrochloride
Catalog No.:BCC5134
CAS No.:224177-60-0
- 3-Epiturraeanthin
Catalog No.:BCN5063
CAS No.:22415-24-3
- Cyclobuxine D
Catalog No.:BCC9221
CAS No.:2241-90-9
- Aristola-1(10),8-dien-2-one
Catalog No.:BCN7608
CAS No.:22391-34-0
- Vardenafil HCl Trihydrate
Catalog No.:BCC2277
CAS No.:224785-90-4
- Erigeside I
Catalog No.:BCN7172
CAS No.:224824-74-2
- N-(1-hydroxy-2-(hydroxymethyl)-4-(4-octylphenyl)butan-2-yl)acetamide
Catalog No.:BCN1483
CAS No.:2249289-10-9
- IDRA 21
Catalog No.:BCC6974
CAS No.:22503-72-6
- Falcarindiol
Catalog No.:BCN5065
CAS No.:225110-25-8
- Cathepsin Inhibitor 1
Catalog No.:BCC4896
CAS No.:225120-65-0
- 8-Hydroxy-9,10-diisobutyryloxythymol
Catalog No.:BCN7786
CAS No.:22518-08-7
- CTU Guanamine
Catalog No.:BCC8921
CAS No.:22535-90-6
- Ocotillone
Catalog No.:BCN5066
CAS No.:22549-21-9
- Robustine
Catalog No.:BCN6653
CAS No.:2255-50-7
- Isocryptotanshinone
Catalog No.:BCN2499
CAS No.:22550-15-8
- Bisabolol Oxide A
Catalog No.:BCC8133
CAS No.:22567-36-8
The characteristics of coronary stenosis in 11,267 patients from Southwest China: a retrospective study.[Pubmed:29019045]
J Thromb Thrombolysis. 2018 Jan;45(1):142-150.
The characteristics of coronary stenosis vary among the different countries or areas. 11,267 patients who have undergone coronary angiography (CAG) from three Southwest China hospitals were investigated. Patient characteristics, coronary stenosis and stent-implant information were recorded and analyzed according to two criteria: "visible stenosis" and ">/= 50% stenosis". The patients who have undergone CAG increased year by year, with patients from 60 to 69 years-old taking the highest ratio (34.69%). Based on the ">/= 50% stenosis" criteria, the stenotic frequency was 40.54% for Southwest China patients getting CAG. Only 8.14% patients suffered >/= 3 stenotic vessels, while 11.58 and 20.82% patients had 2 or 1 stenotic vessel, respectively. However, when using the "visible stenosis" criteria, the stenotic frequency increased to 64.68%. The prevalence of stenosis increased with age based on the "visible stenosis" criteria. There were more male patients with stenosis than female except patients over 80 years old. The stenosis affected almost all main coronary arteries and their branches, with the most affected artery being the left anterior descending artery. There were 3246 cases (28.8%) implanted with 5423 stents with a concurrent age-dependent increasing tendency for stent-implant frequency and average implanted stent number. The numbers of patients who have undergone CAG and suffered from CVD increased rapidly. In these patients, positive rate of CAG was 64.67%, which increased to 72.2% in patients over 60-years old. The incidence of >/= 75% stenosis and multiple stenosis increased with age, however it should be noticed there were 18.93% for >/= 75% stenosis and 19.52% for multiple stenosis in patients under 40 years old.
Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma.[Pubmed:29018077]
Blood. 2017 Nov 30;130(22):2401-2409.
Venetoclax is a selective, orally bioavailable BCL-2 inhibitor that induces cell death in multiple myeloma (MM) cells, particularly in those harboring t(11;14), which express high levels of BCL-2 relative to BCL-XL and MCL-1. In this phase 1 study, patients with relapsed/refractory MM received venetoclax monotherapy. After a 2-week lead-in with weekly dose escalation, daily venetoclax was given at 300, 600, 900, or 1200 mg in dose-escalation cohorts and 1200 mg in the safety expansion. Dexamethasone could be added on progression during treatment. Sixty-six patients were enrolled (30, dose-escalation cohorts; 36, safety expansion). Patients received a median of 5 prior therapies (range, 1-15); 61% were bortezomib and lenalidomide double refractory, and 46% had t(11;14). Venetoclax was generally well tolerated. Most common adverse events included mild gastrointestinal symptoms (nausea [47%], diarrhea [36%], vomiting [21%]). Cytopenias were the most common grade 3/4 events, with thrombocytopenia (32%), neutropenia (27%), anemia (23%), and leukopenia (23%) reported. The overall response rate (ORR) was 21% (14/66), and 15% achieved very good partial response or better (>/=VGPR). Most responses (12/14 [86%]) were reported in patients with t(11;14). In this group, ORR was 40%, with 27% of patients achieving >/=VGPR. Biomarker analysis confirmed that response to venetoclax correlated with higher BCL2:BCL2L1 and BCL2:MCL1 mRNA expression ratios. Venetoclax monotherapy at a daily dose up to 1200 mg has an acceptable safety profile and evidence of single-agent antimyeloma activity in patients with relapsed/refractory MM, predominantly in patients with t(11;14) abnormality and those with a favorable BCL2 family profile. Registered at www.clinicaltrials.gov: #NCT01794520.


