MLCK inhibitor peptide 18Selective inhibitor of myosin light chain kinase CAS# 224579-74-2 |
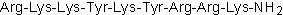
- LDN193189 Hydrochloride
Catalog No.:BCC1695
CAS No.:1062368-62-0
- ASP3026
Catalog No.:BCC1372
CAS No.:1097917-15-1
- AP26113
Catalog No.:BCC1069
CAS No.:1197958-12-5
- CH5424802
Catalog No.:BCC3749
CAS No.:1256580-46-7
- ALK inhibitor 2
Catalog No.:BCC1340
CAS No.:761438-38-4
- (R)-Crizotinib
Catalog No.:BCC1284
CAS No.:877399-52-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 224579-74-2 | SDF | Download SDF |
| PubChem ID | 10102940 | Appearance | Powder |
| Formula | C60H105N23O11 | M.Wt | 1324.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Myosin Light Chain Kinase Inhibitor Peptide 18 | ||
| Solubility | H2O : 50 mg/mL (37.75 mM; Need ultrasonic) | ||
| Sequence | RKKYKYRRK (Modifications: Lys-9 = C-terminal amide) | ||
| Chemical Name | (2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-6-amino-2-[[(2S)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]hexanoyl]amino]hexanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]hexanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]hexanamide | ||
| SMILES | C1=CC(=CC=C1CC(C(=O)NC(CCCCN)C(=O)NC(CC2=CC=C(C=C2)O)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCCN)C(=O)N)NC(=O)C(CCCCN)NC(=O)C(CCCCN)NC(=O)C(CCCN=C(N)N)N)O | ||
| Standard InChIKey | JPOKAKNGULMYHZ-UILVTTEASA-N | ||
| Standard InChI | InChI=1S/C60H105N23O11/c61-27-5-1-13-41(49(66)86)76-51(88)45(17-10-32-74-59(69)70)79-53(90)46(18-11-33-75-60(71)72)81-57(94)48(35-37-21-25-39(85)26-22-37)83-55(92)44(16-4-8-30-64)80-56(93)47(34-36-19-23-38(84)24-20-36)82-54(91)43(15-3-7-29-63)78-52(89)42(14-2-6-28-62)77-50(87)40(65)12-9-31-73-58(67)68/h19-26,40-48,84-85H,1-18,27-35,61-65H2,(H2,66,86)(H,76,88)(H,77,87)(H,78,89)(H,79,90)(H,80,93)(H,81,94)(H,82,91)(H,83,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t40-,41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective competitive inhibitor of myosin light chain kinase (IC50 = 50 nM). Displays 4000-fold selectivity over CaM kinase II and does not inhibit PKA. Cell permeable. |

MLCK inhibitor peptide 18 Dilution Calculator

MLCK inhibitor peptide 18 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MLCK inhibitor peptide 18 is a myosin light chain kinase (MLCK) inhibitor with an IC50 of 50 nM, and inhibits CaM kinase II only at 4000-fold higher concentrations. Sequence: Arg-Lys-Lys-Tyr-Lys-Tyr-Arg-Arg-Lys-NH2.
In Vitro:MLCK inhibitor peptide 18 has a selective effect on peptide substrate utilization by MLCK, does not interfere with kinase activation by CaM, does not have significant inhibitory activity with the closely related CaMPKII, and does not inhibit PKA[1].
- Benfotiamine
Catalog No.:BCC1415
CAS No.:22457-89-2
- Retapamulin
Catalog No.:BCC4837
CAS No.:224452-66-8
- Ginsenoside Rg1
Catalog No.:BCN1066
CAS No.:22427-39-0
- Bayogenin methyl ester
Catalog No.:BCN3722
CAS No.:22425-81-6
- 2,2'-Anhydro-5-methyluridine
Catalog No.:BCC8486
CAS No.:22423-26-3
- Incensole
Catalog No.:BCN3831
CAS No.:22419-74-5
- Siramesine hydrochloride
Catalog No.:BCC5134
CAS No.:224177-60-0
- 3-Epiturraeanthin
Catalog No.:BCN5063
CAS No.:22415-24-3
- Cyclobuxine D
Catalog No.:BCC9221
CAS No.:2241-90-9
- Aristola-1(10),8-dien-2-one
Catalog No.:BCN7608
CAS No.:22391-34-0
- 3,8-Di-O-methylellagic acid
Catalog No.:BCN5062
CAS No.:2239-88-5
- Serratenediol
Catalog No.:BCN5061
CAS No.:2239-24-9
- Gymnemagenin
Catalog No.:BCN7841
CAS No.:22467-07-8
- 18-Norabieta-8,11,13-trien-4-ol
Catalog No.:BCN5064
CAS No.:22478-65-5
- Vardenafil HCl Trihydrate
Catalog No.:BCC2277
CAS No.:224785-90-4
- Erigeside I
Catalog No.:BCN7172
CAS No.:224824-74-2
- N-(1-hydroxy-2-(hydroxymethyl)-4-(4-octylphenyl)butan-2-yl)acetamide
Catalog No.:BCN1483
CAS No.:2249289-10-9
- IDRA 21
Catalog No.:BCC6974
CAS No.:22503-72-6
- Falcarindiol
Catalog No.:BCN5065
CAS No.:225110-25-8
- Cathepsin Inhibitor 1
Catalog No.:BCC4896
CAS No.:225120-65-0
- 8-Hydroxy-9,10-diisobutyryloxythymol
Catalog No.:BCN7786
CAS No.:22518-08-7
- CTU Guanamine
Catalog No.:BCC8921
CAS No.:22535-90-6
- Ocotillone
Catalog No.:BCN5066
CAS No.:22549-21-9
- Robustine
Catalog No.:BCN6653
CAS No.:2255-50-7
Myosin light chain kinase mediates intestinal barrier dysfunction via occludin endocytosis during anoxia/reoxygenation injury.[Pubmed:27760753]
Am J Physiol Cell Physiol. 2016 Dec 1;311(6):C996-C1004.
Intestinal anoxia/reoxygenation (A/R) injury induces loss of barrier function followed by epithelial repair. Myosin light chain kinase (MLCK) has been shown to alter barrier function via regulation of interepithelial tight junctions, but has not been studied in intestinal A/R injury. We hypothesized that A/R injury would disrupt tight junction barrier function via MLCK activation and myosin light chain (MLC) phosphorylation. Caco-2BBe1 monolayers were subjected to anoxia for 2 h followed by reoxygenation in 21% O2, after which barrier function was determined by measuring transepithelial electrical resistance (TER) and FITC-dextran flux. Tight junction proteins and MLCK signaling were assessed by Western blotting, real-time PCR, or immunofluorescence microscopy. The role of MLCK was further investigated with select inhibitors (ML-7 and peptide 18) by using in vitro and ex vivo models. Following A/R injury, there was a significant increase in paracellular permeability compared with control cells, as determined by TER and dextran fluxes (P < 0.05). The tight junction protein occludin was internalized during A/R injury and relocalized to the region of the tight junction after 4 h of recovery. MLC phosphorylation was significantly increased by A/R injury (P < 0.05), and treatment with the MLCK inhibitor peptide 18 attenuated the increased epithelial monolayer permeability and occludin endocytosis caused by A/R injury. Application of MLCK inhibitors to ischemia-injured porcine ileal mucosa induced significant increases in TER and reduced mucosal-to-serosal fluxes of (3)H-labeled mannitol. These data suggest that MLCK-induced occludin endocytosis mediates intestinal epithelial barrier dysfunction during A/R injury. Our results also indicate that MLCK-dependent occludin regulation may be a target for the therapeutic treatment of ischemia/reperfusion injury.
A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease.[Pubmed:12105845]
Gastroenterology. 2002 Jul;123(1):163-72.
BACKGROUND & AIMS: Maintenance of the mucosal barrier is a critical function of intestinal epithelia. Myosin regulatory light chain (MLC) phosphorylation is a common intermediate in the pathophysiologic regulation of this barrier. The aim of this study was to determine whether a membrane permeant inhibitor of MLC kinase (PIK) could inhibit intracellular MLC kinase and regulate paracellular permeability. METHODS: Recombinant MLC and Caco-2 MLC kinase were used for kinase assays. T84 and Caco-2 monolayers were treated with enteropathogenic Escherichia coli (EPEC) or tumor necrosis factor (TNF)-alpha and interferon (IFN)-gamma to induce barrier dysfunction. RESULTS: PIK inhibited MLC kinase in vitro and was able to cross cell membranes and concentrate at the perijunctional actomyosin ring. Consistent with these properties, apical addition of PIK reduced intracellular MLC phosphorylation by 22% +/- 2%, increased transepithelial resistance (TER) by 50% +/- 1%, and decreased paracellular mannitol flux rates by 5.2 +/- 0.2-fold. EPEC infection induced TER decreases of 37% +/- 6% that were limited to 16% +/- 5% by PIK. TNF-alpha and IFN-gamma induced TER decreases of 22% +/- 3% that were associated with a 172% +/- 1% increase in MLC phosphorylation. Subsequent PIK addition caused MLC phosphorylation to decrease by 25% +/- 4% while TER increased to 97% +/- 6% of control. CONCLUSIONS: PIK can prevent TER defects induced by EPEC and reverse MLC phosphorylation increases and TER decreases induced by TNF-alpha and IFN-gamma. The data also suggest that TNF-alpha and IFN-gamma regulate TER, at least in part, via the perijunctional cytoskeleton. Thus, PIK may be the prototype for a new class of targeted therapeutic agents that can restore barrier function in intestinal disease states.
Identification of novel classes of protein kinase inhibitors using combinatorial peptide chemistry based on functional genomics knowledge.[Pubmed:10072688]
J Med Chem. 1999 Mar 11;42(5):910-9.
A discovery approach based on an intramolecular inhibitory mechanism was applied to a prototype calmodulin (CaM)-regulated protein kinase in order to demonstrate a proof-of-principle for the development of selective inhibitors. The overall approach used functional genomics analysis of myosin light chain kinase (MLCK) to identify short autoinhibitory sequences that lack CaM recognition activity, followed by recursive combinatorial peptide library production and comparative activity screens. Peptide 18 (Arg-Lys-Lys-Tyr-Lys-Tyr-Arg-Arg-Lys-NH2), one of several selective inhibitors discovered, has an IC50 = 50 nM for MLCK, inhibits CaM kinase II only at 4000-fold higher concentrations, and does not inhibit cyclic AMP-dependent protein kinase. Analogues of peptide 18 containing conformationally constrained cis-4-aminocyclohexanecarboxylic acid retained affinity and selectivity. The inhibitors add to the armamentarium available for the deconvolution of complex signal transduction pathways and their relationship to homeostasis and disease, and the approach is potentially applicable to enzymes in which the catalytic and regulatory domains are found within the same open reading frame of a cDNA.


