BenfotiamineDrug for painful nerve condition CAS# 22457-89-2 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 22457-89-2 | SDF | Download SDF |
| PubChem ID | 3032771 | Appearance | Powder |
| Formula | C19H23N4O6PS | M.Wt | 466.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | S-Benzoylthiamine O-monophosphate | ||
| Solubility | DMSO : ≥ 50 mg/mL (107.19 mM) H2O : 0.67 mg/mL (1.44 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
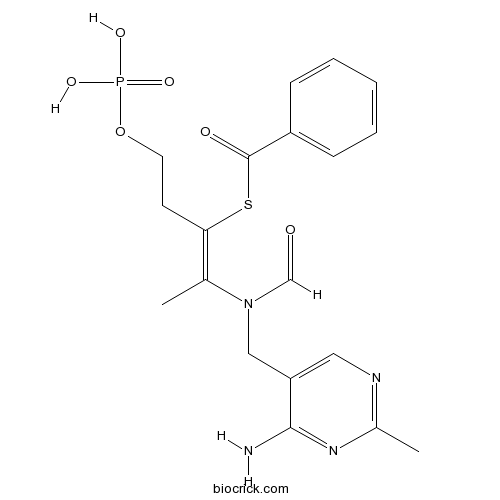
| Chemical Name | S-[(Z)-2-[(4-amino-2-methylpyrimidin-5-yl)methyl-formylamino]-5-phosphonooxypent-2-en-3-yl] benzenecarbothioate | ||
| SMILES | CC1=NC=C(C(=N1)N)CN(C=O)C(=C(CCOP(=O)(O)O)SC(=O)C2=CC=CC=C2)C | ||
| Standard InChIKey | BTNNPSLJPBRMLZ-LGMDPLHJSA-N | ||
| Standard InChI | InChI=1S/C19H23N4O6PS/c1-13(23(12-24)11-16-10-21-14(2)22-18(16)20)17(8-9-29-30(26,27)28)31-19(25)15-6-4-3-5-7-15/h3-7,10,12H,8-9,11H2,1-2H3,(H2,20,21,22)(H2,26,27,28)/b17-13- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Benfotiamine is a synthetic S-acyl derivative of thiamine (vitamin B1); an antioxidant dietary supplement.
IC50 value:
Target:
Benfotiamine, the lipid-soluble thiamine derivative used as a treatment for diabetic neuropathy, can inhibit three major pathways(the hexosamine pathway, the advanced glycation end product (AGE) formation pathway and the diacylglycerol (DAG)?protein kinase C (PKC) pathway)of hyperglycemic damage and prevent experimental diabetic retinopathy. Benfotiamine is a synthetic S-acyl derivative of thiamine (vitamin B1) for treating sciatica and other painful nerve conditions. More effective at increasing thiamin levels in blood and tissues than water-soluble salts like the previous vitamin B1. References: | |||||

Benfotiamine Dilution Calculator

Benfotiamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1439 mL | 10.7193 mL | 21.4385 mL | 42.8771 mL | 53.5963 mL |
| 5 mM | 0.4288 mL | 2.1439 mL | 4.2877 mL | 8.5754 mL | 10.7193 mL |
| 10 mM | 0.2144 mL | 1.0719 mL | 2.1439 mL | 4.2877 mL | 5.3596 mL |
| 50 mM | 0.0429 mL | 0.2144 mL | 0.4288 mL | 0.8575 mL | 1.0719 mL |
| 100 mM | 0.0214 mL | 0.1072 mL | 0.2144 mL | 0.4288 mL | 0.536 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Benfotiamine, the lipid-soluble thiamine derivative used as a treatment for diabetic neuropathy, can inhibit three major pathways(the hexosamine pathway, the advanced glycation end product (AGE) formation pathway and the diacylglycerol (DAG)?protein kinase C (PKC) pathway)of hyperglycemic damage and prevent experimental diabetic retinopathy. Benfotiamine is a synthetic S-acyl derivative of thiamine (vitamin B1) for treating sciatica and other painful nerve conditions. More effective at increasing thiamin levels in blood and tissues than water-soluble salts like the previous vitamin B1.
- Retapamulin
Catalog No.:BCC4837
CAS No.:224452-66-8
- Ginsenoside Rg1
Catalog No.:BCN1066
CAS No.:22427-39-0
- Bayogenin methyl ester
Catalog No.:BCN3722
CAS No.:22425-81-6
- 2,2'-Anhydro-5-methyluridine
Catalog No.:BCC8486
CAS No.:22423-26-3
- Incensole
Catalog No.:BCN3831
CAS No.:22419-74-5
- Siramesine hydrochloride
Catalog No.:BCC5134
CAS No.:224177-60-0
- 3-Epiturraeanthin
Catalog No.:BCN5063
CAS No.:22415-24-3
- Cyclobuxine D
Catalog No.:BCC9221
CAS No.:2241-90-9
- Aristola-1(10),8-dien-2-one
Catalog No.:BCN7608
CAS No.:22391-34-0
- 3,8-Di-O-methylellagic acid
Catalog No.:BCN5062
CAS No.:2239-88-5
- Serratenediol
Catalog No.:BCN5061
CAS No.:2239-24-9
- Pedalitin
Catalog No.:BCN3954
CAS No.:22384-63-0
- MLCK inhibitor peptide 18
Catalog No.:BCC5828
CAS No.:224579-74-2
- Gymnemagenin
Catalog No.:BCN7841
CAS No.:22467-07-8
- 18-Norabieta-8,11,13-trien-4-ol
Catalog No.:BCN5064
CAS No.:22478-65-5
- Vardenafil HCl Trihydrate
Catalog No.:BCC2277
CAS No.:224785-90-4
- Erigeside I
Catalog No.:BCN7172
CAS No.:224824-74-2
- N-(1-hydroxy-2-(hydroxymethyl)-4-(4-octylphenyl)butan-2-yl)acetamide
Catalog No.:BCN1483
CAS No.:2249289-10-9
- IDRA 21
Catalog No.:BCC6974
CAS No.:22503-72-6
- Falcarindiol
Catalog No.:BCN5065
CAS No.:225110-25-8
- Cathepsin Inhibitor 1
Catalog No.:BCC4896
CAS No.:225120-65-0
- 8-Hydroxy-9,10-diisobutyryloxythymol
Catalog No.:BCN7786
CAS No.:22518-08-7
- CTU Guanamine
Catalog No.:BCC8921
CAS No.:22535-90-6
- Ocotillone
Catalog No.:BCN5066
CAS No.:22549-21-9
Long-Term Cognitive Improvement After Benfotiamine Administration in Patients with Alzheimer's Disease.[Pubmed:27696179]
Neurosci Bull. 2016 Dec;32(6):591-596.
To date, we still lack disease-modifying therapies for Alzheimer's disease (AD). Here, we report that long-term administration of Benfotiamine improved the cognitive ability of patients with AD. Five patients with mild to moderate AD received oral Benfotiamine (300 mg daily) over 18 months. All patients were examined by positron emission tomography with Pittsburgh compound B (PiB-PET) and exhibited positive imaging with beta-amyloid deposition, and three received PiB-PET imaging at follow-up. The five patients exhibited cognitive improvement as assayed by the Mini-Mental Status Examination (MMSE) with an average increase of 3.2 points at month 18 of Benfotiamine administration. The three patients who received follow-up PiB-PET had a 36.7% increase in the average standardized uptake value ratio in the brain compared with that in the first scan. Importantly, the MMSE scores of these three had an average increase of 3 points during the same period. Benfotiamine significantly improved the cognitive abilities of mild to moderate AD patients independently of brain amyloid accumulation. Our study provides new insight to the development of disease-modifying therapy.
Protective effect of treatment with thiamine or benfotiamine on liver oxidative damage in rat model of acute ethanol intoxication.[Pubmed:27545821]
Life Sci. 2016 Oct 1;162:21-4.
AIMS: The aim of this study was to evaluate possible beneficial effects of treatment with thiamine or Benfotiamine in an animal model of acute ethanol intoxication. MAIN METHODS: Thirty male Wistar rats were separated at random into three groups of 10 animals each: Ethanol (E), Ethanol treated with thiamine (T) and Ethanol treated with Benfotiamine (BE). Rats were gavaged with single dose of ethanol (5g/kg, 40% v:v). After 30min of ethanol gavage the animals were treated with thiamine or Benfotiamine. Six hours after first gavage, the animals were euthanized and blood and liver samples were collected for ethanol and oxidative stress biomarkers quantification. KEY FINDINGS: Serum ethanol levels were higher in animals treated with thiamine or Benfotiamine while hepatic alcohol levels were higher in animals of the group treated with Benfotiamine comparing to controls or thiamine treated groups. The lipid peroxidation biomarkers were diminished for the groups treated with thiamine or Benfotiamine comparing to E animals. Concerning protein oxidative damage parameters, they were enhanced for animals treated with Benfotiamine in relation to other groups. SIGNIFICANCE: In conclusion, the treatment with thiamine or Benfotiamine even 30min after the massive dose of ethanol has proven to be beneficial against liver damage. Improved results were obtained with Benfotiamine in relation to oxidative damage from aqueous compartments.
Chromatographic Analysis of a Multicomponent Mixture of B1, B6, B12, Benfotiamine, and Diclofenac; Part I: HPLC and UPLC Methods for the Simultaneous Quantification of These Five Components in Tablets and Capsules.[Pubmed:27697097]
J AOAC Int. 2016 Nov 1;99(6):1513-1521.
New, simple, highly sensitive, precise, and accurate gradient reversed-phase chromatographic methods were developed using HPLC and ultra-HPLC (UPLC) systems for the determination of five components, namely thiamine, pyridoxine, cyanocobalamin, Benfotiamine, and diclofenac in tablets and capsules. The methods were compared for their efficiency in the separation and determination of these five compounds using two different C18 columns (250 x 4.6 mm, 5 mum; and 100 x 4.6 mm, 2.6 mum) for HPLC and UPLC, respectively. Chromatographic separation was performed with a mobile phase containing acetonitrile and 0.025 M phosphate buffer (pH 3.5), with a gradient program and a flow rate of 1.5 and 1.0 mL/min for both methods, respectively. The methods were validated according to International Conference on Harmonization guidelines. Linearity was achieved in the range of 5.00 to 150.00 mug/mL for each of the five compounds. Ruggedness and intermediate precision were confirmed by different analysts on different columns on different days. Moreover, the components were subjected to an accelerated stability study under acidic, alkaline, and oxidative stress conditions and no interfering peaks were observed. The five compounds were efficiently separated in <20 min by HPLC, whereas for UPLC, separation was achieved in <8 min, which dramatically decreased the consumption of organic solvents.
Thiamine and benfotiamine improve cognition and ameliorate GSK-3beta-associated stress-induced behaviours in mice.[Pubmed:27825907]
Prog Neuropsychopharmacol Biol Psychiatry. 2017 Apr 3;75:148-156.
Thiamine (vitamin B1) deficiency in the brain has been implicated in the development of dementia and symptoms of depression. Indirect evidence suggests that thiamine may contribute to these pathologies by controlling the activities of glycogen synthase kinase (GSK)-3beta. While decreased GSK-3beta activity appears to impair memory, increased GSK-3beta activity is associated with the distressed/depressed state. However, hitherto direct evidence for the effects of thiamine on GSK-3beta function has not been reported. Here, we administered thiamine or, the more bioavailable precursor, Benfotiamine at 200mg/kg/day for 2weeks to C57BL/6J mice, to determine whether treatment might affect behaviours that are known to be sensitive to GSK-3beta activity and whether such administration impacts on GSK-3beta expression within the brain. The mice were tested in models of contextual conditioning and extinction, a 5-day rat exposure stress test, and a modified swim test with repeated testing. The tricyclic antidepressant imipramine (7.5mg/kg/day), was administered as a positive control for the effects of thiamine or Benfotiamine. As for imipramine, both compounds inhibited the upregulation of GSK-3beta induced by predator stress or repeated swimming, and reduced floating scores and the predator stress-induced behavioural changes in anxiety and exploration. Coincident, thiamine and Benfotiamine improved learning and extinction of contextual fear, and the acquisition of the step-down avoidance task. Our data indicate that thiamine and Benfotiamine have antidepressant/anti-stress effects in naive animals that are associated with reduced GSK-3beta expression and conditioning of adverse memories. Thus thiamine and Benfotiamine may modulate GSK-3beta functions in a manner that is dependent on whether the contextual conditioning is adaptive or maladaptive.


