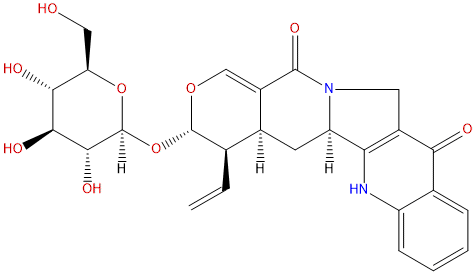
3-epi-PumilosideCAS# 126624-21-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 126624-21-3 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C26H28N2O9 | M.Wt | 512.51 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3-epi-Pumiloside Dilution Calculator

3-epi-Pumiloside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9512 mL | 9.7559 mL | 19.5118 mL | 39.0236 mL | 48.7795 mL |
| 5 mM | 0.3902 mL | 1.9512 mL | 3.9024 mL | 7.8047 mL | 9.7559 mL |
| 10 mM | 0.1951 mL | 0.9756 mL | 1.9512 mL | 3.9024 mL | 4.878 mL |
| 50 mM | 0.039 mL | 0.1951 mL | 0.3902 mL | 0.7805 mL | 0.9756 mL |
| 100 mM | 0.0195 mL | 0.0976 mL | 0.1951 mL | 0.3902 mL | 0.4878 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2'-Deoxyadenosine monohydrate
Catalog No.:BCX1578
CAS No.:16373-93-6
- Uvarigranol F
Catalog No.:BCX1577
CAS No.:176598-10-0
- 3-Hydroxy-3-methylpentanedioic acid
Catalog No.:BCX1576
CAS No.:503-49-1
- Gelsemicine
Catalog No.:BCX1575
CAS No.:6887-28-1
- Hydroxyl-γ-isosanshool
Catalog No.:BCX1574
CAS No.:127514-62-9
- Uridine diphosphate xylose
Catalog No.:BCX1573
CAS No.:3616-06-6
- Uridine diphosphate rhamnose
Catalog No.:BCX1572
CAS No.:1955-26-6
- Trisodium UDP-glucuronic acid
Catalog No.:BCX1571
CAS No.:63700-19-6
- Escin Ie
Catalog No.:BCX1570
CAS No.:1613506-26-5
- α-Sanshool
Catalog No.:BCX1569
CAS No.:504-97-2
- 18α-Glycyrrhetinic acid Monoglucuronide
Catalog No.:BCX1568
CAS No.:400870-85-1
- Pinocembrin 7-O-[4'',6''-hexahydroxydiphenoyl]-β-D-glucoside
Catalog No.:BCX1567
CAS No.:205370-58-7
- Saikogenin A
Catalog No.:BCX1580
CAS No.:5092-09-1
- Methyl pentadecanoate
Catalog No.:BCX1581
CAS No.:7132-64-1
- Phosphatidylserine
Catalog No.:BCX1582
CAS No.:51446-62-9
- 4-(3-Oxobutyl)phenyl 6-O-[(2E)-3-phenyl-2-propenoyl]-2-O-(3,4,5-trihydroxybenzoyl)-β-D-glucopyranoside
Catalog No.:BCX1583
CAS No.:356517-93-6
- Bacdanol
Catalog No.:BCX1584
CAS No.:28219-61-6
- β-Sanshool
Catalog No.:BCX1585
CAS No.:10076-00-3
- Timosaponin H1
Catalog No.:BCX1586
CAS No.:288142-06-3
- Trikamsteroside A
Catalog No.:BCX1587
CAS No.:942916-76-9
- Thalictricoside
Catalog No.:BCX1588
CAS No.:649758-25-8
- HuangjiangSu A
Catalog No.:BCX1589
CAS No.:1026020-27-8
- Telephiose A
Catalog No.:BCX1590
CAS No.:297749-29-2
- Wuweizidilactone A
Catalog No.:BCX1591
CAS No.:945610-99-1
Anti-inflammatory effect of the six compounds isolated from Nauclea officinalis Pierrc ex Pitard, and molecular mechanism of strictosamide via suppressing the NF-kappaB and MAPK signaling pathway in LPS-induced RAW 264.7 macrophages.[Pubmed:27989509]
J Ethnopharmacol. 2017 Jan 20;196:66-74.
ETHNOPHARMACOLOGICAL RELEVANCE: Nauclea officinalis Pierrc ex Pitard. is a Chinese medicinal herb that contains high level of alkaloids which is the most abundant and active constituent. Strictosamide isolated from Nauclea officinalis Pierrc ex Pitard. showed significant effects on inflammatory response, compared with pumiloside, 3-epi-Pumiloside, vincosamide, 3alpha,5alpha-tetrahydrodeoxycordifoline lactam and naucleamide A-10-O-beta-D-glucopyranoside of this plant. AIM OF STUDY: we investigated the biological activities of the six compounds mentioned-above, and the underlying molecular mechanism exerted by the most potent one, strictosamide. MATERIALS AND METHODS: The effects of strictosamide and other five compounds on the inhibitory activity of nitric oxide (NO) were screened by Griess test. The contents of tumor necrosis factor-alpha (TNF-alpha) and interleukin-1beta (IL-1beta) in media were detected by using Enzyme-linked immunosorbent (ELISA) kits. The effects on the mRNA expression of nitric oxide synthase (iNOS), TNF-alpha and IL-1beta of strictosamide were further investigated by RT-qPCR. Western blot assay was conducted to illustrate the effects of strictosamide on iNOS and phosphorylation of p65, inhibitor of NF-kappaB (IkappaB)-alpha, IkappaB-kinase (IKK)-alpha as well as p-extracellular signal-regulated kinase (ERK), p-c-jun N-terminal kinase (JNK) and p-p38 in the protein levels. RESULTS: Strictosamide potently suppressed the productions of NO, TNF-alpha and IL-1beta in LPS-induced RAW 264.7 macrophages, and it dose-dependently alleviated the LPS-simulated protein level of iNOS as well as the mRNA expressions of iNOS, TNF-alpha and IL-1beta. In addition, molecular data revealed that strictosamide markedly decreased the expressions of p-p65, p-IkappaBalpha and p-IKKalpha. Furthermore, strictosamide significantly attenuated LPS-induced the phosphorylation of p38, ERK and JNK. CONCLUSIONS: At present study, the results indicated that the anti-inflammatory activity of strictosamide was associated with the restraint of NO, TNF-alpha and IL-1beta via negative regulation of both NF-kappaB and mitogen-activated protein kinases (MAPKs) in LPS-induced RAW 264.7 cells.
New indole glucosides as biosynthetic intermediates of camptothecin from the fruits of Camptotheca acuminata.[Pubmed:25771119]
Fitoterapia. 2015 Jun;103:1-8.
Six new indole glucosides (1-6) and fifteen known alkaloids (7-21) were isolated from the fruit of Camptotheca acuminata. The planar structures of 1-6 were determined on the basis of spectroscopic data analysis and their absolute configurations were established by CD. The isolated indole glucosides showed a clear biosynthetic pathway of camptothecin (7), which started from tryptamine and secologanin and was proposed by synthetic chemists previously. Particularly, compound 1 supplemented the process of the transformation from pumiloside (20) or 3-epi-Pumiloside (21) to camptothecin (7). In addition, camptothecin 10-O-beta-D-glucopyranoside (13) and norcamptothecin (17), synthesized in the early structural modification of 7, were first isolated from the natural resources. The new compounds 1-6 were screened for their in vitro cytotoxicity but they did not show any exciting result.
Simultaneous determination of six alkaloid components in rat plasma and its application to pharmacokinetic study of Danmu preparations by an ultra fast liquid chromatography-electrospray ionization-tandem mass spectrometry.[Pubmed:25612771]
J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Mar 1;983-984:10-7.
Danmu injection and Danmu tablet are two widely used traditional Chinese medicine made of Nauclea officinalis (commonly known as Danmu), in which the alkaloids are the major active substances. In this paper, an ultra fast liquid chromatography-tandem mass spectrometry (UFLC-MS/MS) method was developed for simultaneous determination and the pharmacokinetic characteristics study of six main active alkaloids (naucleamide A-10-O-beta-d-glucopyranosid, naucleamide G, pumiloside, 3-epi-Pumiloside, strictosamide and vincosamide) of the two above-mentioned Danmu preparations in rat plasma. In the course of the experiment, following sample preparation by protein precipitation with methanol-ethyl acetate (2:1, v/v), the nitrogen-dried extraction was reconstituted in methanol and assayed on a C18 column using a gradient elution program with mobile phase consisting of acetonitrile and water containing 0.1% formic acid. The MS detection was performed in positive ionization mode with selected ion transitions. The established method was fully validated and proved to be sensitive and specific with lower limits of quantification (LLOQs) all less than 0.32ng/mL in rat plasma and matrix effects ranged from 88.87 to 108.27%. Good linearities of six alkaloids were obtained in respective concentration ranges (r(2)>0.995). The average extract recoveries for each compound at three quality control concentration levels were no less than 79.70%, and the precision and accuracy were within the acceptable limits. The validated method was successfully applied to the pharmacokinetic study of six alkaloid components of Danmu injection and tablet in rat plasma. The obtained results may be helpful to reveal the action mechanism and guide the clinical application of Danmu preparations.
[Chemical constituents of Nauclea officinalis].[Pubmed:23672026]
Yao Xue Xue Bao. 2013 Feb;48(2):276-80.
In order to study the chemical constituents in the water extract of the stem of Nauclea officinalis, column chromatography over D101 macroporous resin and silica gel and an automatic purification system were used to isolate and purify the chemical constituents from the extract. Nine compounds were obtained. By analysis of the physicochemical properties and spectral data, their structures were identified as naucleamide G (1), 3, 4-dimethoxyphenol-beta-D-apiofuranosyl (1-->6)-beta-D-glucopyranoside (2), kelampayoside A (3), 3alpha, 5alpha-tetrahydrodeoxycordifoline lactam (4), naucleamide A-10-O-beta-D-glucopyranoside (5), pumiloside (6), 3-epi-Pumiloside (7), strictosamide (8) and vincosamide (9), separately. Among them, compound 1 is a new compound, compound 2 was found in plants of the genus Nauclea for the first time, and compounds 3 and 4 were isolated from this plant for the first time.


