GelsemicineCAS# 6887-28-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6887-28-1 | SDF | Download SDF |
| PubChem ID | 5462428.0 | Appearance | Powder |
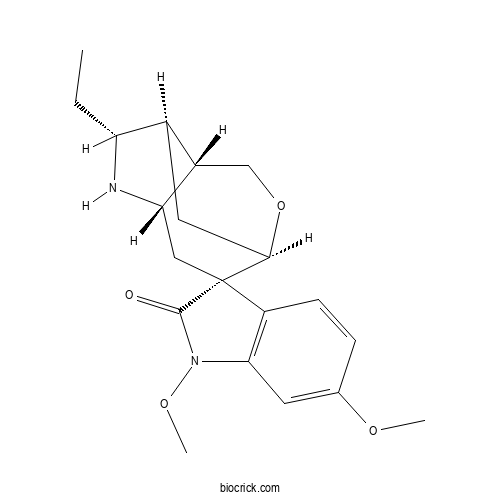
| Formula | C20H26N2O4 | M.Wt | 358.44 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2S,4S,6R,7R,8S)-6-ethyl-1',6'-dimethoxyspiro[10-oxa-5-azatricyclo[5.3.1.04,8]undecane-2,3'-indole]-2'-one | ||
| SMILES | CCC1C2CC3C4(CC(C2CO3)N1)C5=C(C=C(C=C5)OC)N(C4=O)OC | ||
| Standard InChIKey | RIHQHYIWKHVLRH-XKTBTPLDSA-N | ||
| Standard InChI | InChI=1S/C20H26N2O4/c1-4-15-12-8-18-20(9-16(21-15)13(12)10-26-18)14-6-5-11(24-2)7-17(14)22(25-3)19(20)23/h5-7,12-13,15-16,18,21H,4,8-10H2,1-3H3/t12-,13+,15-,16+,18-,20+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Gelsemicine Dilution Calculator

Gelsemicine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7899 mL | 13.9493 mL | 27.8987 mL | 55.7973 mL | 69.7467 mL |
| 5 mM | 0.558 mL | 2.7899 mL | 5.5797 mL | 11.1595 mL | 13.9493 mL |
| 10 mM | 0.279 mL | 1.3949 mL | 2.7899 mL | 5.5797 mL | 6.9747 mL |
| 50 mM | 0.0558 mL | 0.279 mL | 0.558 mL | 1.1159 mL | 1.3949 mL |
| 100 mM | 0.0279 mL | 0.1395 mL | 0.279 mL | 0.558 mL | 0.6975 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hydroxyl-γ-isosanshool
Catalog No.:BCX1574
CAS No.:127514-62-9
- Uridine diphosphate xylose
Catalog No.:BCX1573
CAS No.:3616-06-6
- Uridine diphosphate rhamnose
Catalog No.:BCX1572
CAS No.:1955-26-6
- Trisodium UDP-glucuronic acid
Catalog No.:BCX1571
CAS No.:63700-19-6
- Escin Ie
Catalog No.:BCX1570
CAS No.:1613506-26-5
- α-Sanshool
Catalog No.:BCX1569
CAS No.:504-97-2
- 18α-Glycyrrhetinic acid Monoglucuronide
Catalog No.:BCX1568
CAS No.:400870-85-1
- Pinocembrin 7-O-[4'',6''-hexahydroxydiphenoyl]-β-D-glucoside
Catalog No.:BCX1567
CAS No.:205370-58-7
- Lutein distearate
Catalog No.:BCX1566
CAS No.:86852-15-5
- Lutein myristate palmitate
Catalog No.:BCX1565
CAS No.:104784-49-8
- Lutein dimyristate
Catalog No.:BCX1564
CAS No.:86853-02-3
- Lutein monomyristate
Catalog No.:BCX1563
CAS No.:56842-49-0
- 3-Hydroxy-3-methylpentanedioic acid
Catalog No.:BCX1576
CAS No.:503-49-1
- Uvarigranol F
Catalog No.:BCX1577
CAS No.:176598-10-0
- 2'-Deoxyadenosine monohydrate
Catalog No.:BCX1578
CAS No.:16373-93-6
- 3-epi-Pumiloside
Catalog No.:BCX1579
CAS No.:126624-21-3
- Saikogenin A
Catalog No.:BCX1580
CAS No.:5092-09-1
- Methyl pentadecanoate
Catalog No.:BCX1581
CAS No.:7132-64-1
- Phosphatidylserine
Catalog No.:BCX1582
CAS No.:51446-62-9
- 4-(3-Oxobutyl)phenyl 6-O-[(2E)-3-phenyl-2-propenoyl]-2-O-(3,4,5-trihydroxybenzoyl)-β-D-glucopyranoside
Catalog No.:BCX1583
CAS No.:356517-93-6
- Bacdanol
Catalog No.:BCX1584
CAS No.:28219-61-6
- β-Sanshool
Catalog No.:BCX1585
CAS No.:10076-00-3
- Timosaponin H1
Catalog No.:BCX1586
CAS No.:288142-06-3
- Trikamsteroside A
Catalog No.:BCX1587
CAS No.:942916-76-9
Targeted Isolation of Hemitheion from Mostuea brunonis, a Proposed Biosynthetic Intermediate of Theionbrunonines.[Pubmed:33825474]
J Nat Prod. 2021 Apr 23;84(4):1409-1413.
Hemitheion (1), a new sulfur-containing vobasane-type indole alkaloid, was isolated, together with three known compounds, vobasine (2), gelsedine (3), and Gelsemicine (4), from the alkaloid extract of the stems of Mostuea brunonis Didr. (Gelsemiaceae). Compound 1 could be straightforwardly isolated. Its structure was elucidated by a combination of spectroscopic methods. Besides corresponding to a formerly postulated biosynthetic intermediate toward theionbrunonines, hemitheion (1) stands among the few monomeric vobasanes lacking an oxygen at C-3. Hemitheion (1) showed moderate antiplasmodial activity in the micromolar range against the strain FcB1 of Plasmodium falciparum and no cytotoxic activity against the MRC-5 cell line at 20 muM.
Gelsemium analgesia and the spinal glycine receptor/allopregnanolone pathway.[Pubmed:25447163]
Fitoterapia. 2015 Jan;100:35-43.
Gelsemium, a small genus of flowering plant from the family Loganiaceae, comprises five species including the popular Gelsemium sempervirens Ait. and Gelsemium elegans Benth., which are indigenous to North America and China/East Asia, respectively. Approximately 120 alkaloids have been isolated and identified from Gelsemium, with the predominant indole alkaloids including gelsemine, koumine, Gelsemicine, gelsenicine, gelsedine, sempervirine, koumidine, koumicine and humantenine. Gelsemine is the principal active alkaloid in G. sempervirens Ait., and koumine and gelsemine are the most and second-most dominant alkaloids in G. elegans Benth. Gelsemium extract and its active alkaloids serve a variety of biological functions, including neurobiological, immunosuppressive and antitumor effects, and have traditionally been used to treat pain, neuralgia, anxiety, insomnia, asthma, respiratory ailments and cancers. This review focuses on animal-based studies of Gelsemium as a pain treatment and its mechanism of action. In contrast to morphine, when administered intrathecally and systemically, koumine, gelsemine and gelsenicine have marked antinociception in inflammatory, neuropathic and bone cancer pains without inducing antinociceptive tolerance. Gelsemium and its active alkaloids may produce antinociception by activating the spinal alpha3 glycine/allopregnanolone pathway. The results of this review support the clinical use of Gelsemium and suggest that its active alkaloids may be developed to treat intractable and other types of pain, preferably after chemical modification. However, Gelsemium is a known toxic plant, and its toxicity limits its appropriate dosage and clinical use. To avoid or decrease the side/toxic effects of Gelsemium, an individual monomer of highly potent alkaloids must be selected, or alkaloids that exhibit greater alpha3 glycine receptor selectivity may be discovered or modified.
Isolation of gelsedine-type indole alkaloids from Gelsemium elegans and evaluation of the cytotoxic activity of gelsemium alkaloids for A431 epidermoid carcinoma cells.[Pubmed:16643063]
J Nat Prod. 2006 Apr;69(4):715-8.
Four new gelsedine-type indole alkaloids (1-4) were isolated from the leaves of Gelsemium elegans, together with 11 known alkaloids. The structures were determined as 14-acetoxygelsenicine (1), 14-acetoxy-15-hydroxygelsenicine (2), 14-hydroxy-19-oxogelsenicine (3), and 14-acetoxygelselegine (4), respectively, by spectroscopic analysis. The cytotoxic effects of 14 Gelsemium alkaloids including two new compounds (1, 2) were evaluated using the A431 human epidermoid carcinoma cell line. Of these, the gelsedine-type alkaloids 14-acetoxy15-hydroxygelsenicine (2) [corrected] 14,15-dihydroxygelsenicine (5), gelsedine (7), and Gelsemicine (8) showed potent cytotoxic effects.


