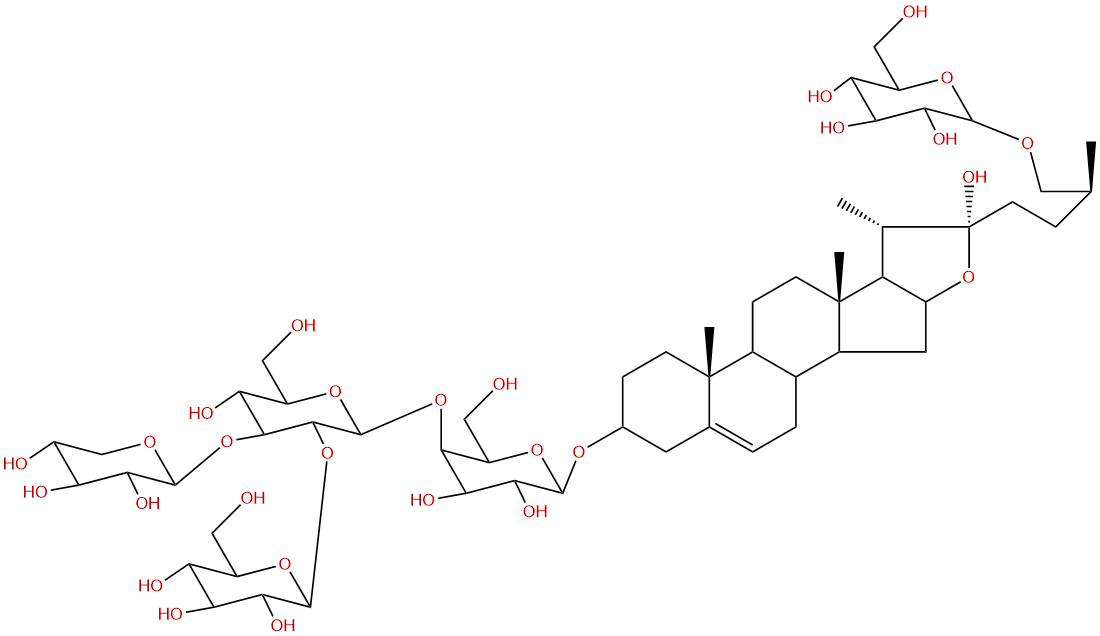
Timosaponin H1CAS# 288142-06-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 288142-06-3 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C56H92O28 | M.Wt | 1213.32 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Timosaponin H1 Dilution Calculator

Timosaponin H1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.8242 mL | 4.1209 mL | 8.2418 mL | 16.4837 mL | 20.6046 mL |
| 5 mM | 0.1648 mL | 0.8242 mL | 1.6484 mL | 3.2967 mL | 4.1209 mL |
| 10 mM | 0.0824 mL | 0.4121 mL | 0.8242 mL | 1.6484 mL | 2.0605 mL |
| 50 mM | 0.0165 mL | 0.0824 mL | 0.1648 mL | 0.3297 mL | 0.4121 mL |
| 100 mM | 0.0082 mL | 0.0412 mL | 0.0824 mL | 0.1648 mL | 0.206 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- β-Sanshool
Catalog No.:BCX1585
CAS No.:10076-00-3
- Bacdanol
Catalog No.:BCX1584
CAS No.:28219-61-6
- 4-(3-Oxobutyl)phenyl 6-O-[(2E)-3-phenyl-2-propenoyl]-2-O-(3,4,5-trihydroxybenzoyl)-β-D-glucopyranoside
Catalog No.:BCX1583
CAS No.:356517-93-6
- Phosphatidylserine
Catalog No.:BCX1582
CAS No.:51446-62-9
- Methyl pentadecanoate
Catalog No.:BCX1581
CAS No.:7132-64-1
- Saikogenin A
Catalog No.:BCX1580
CAS No.:5092-09-1
- 3-epi-Pumiloside
Catalog No.:BCX1579
CAS No.:126624-21-3
- 2'-Deoxyadenosine monohydrate
Catalog No.:BCX1578
CAS No.:16373-93-6
- Uvarigranol F
Catalog No.:BCX1577
CAS No.:176598-10-0
- 3-Hydroxy-3-methylpentanedioic acid
Catalog No.:BCX1576
CAS No.:503-49-1
- Gelsemicine
Catalog No.:BCX1575
CAS No.:6887-28-1
- Hydroxyl-γ-isosanshool
Catalog No.:BCX1574
CAS No.:127514-62-9
- Trikamsteroside A
Catalog No.:BCX1587
CAS No.:942916-76-9
- Thalictricoside
Catalog No.:BCX1588
CAS No.:649758-25-8
- HuangjiangSu A
Catalog No.:BCX1589
CAS No.:1026020-27-8
- Telephiose A
Catalog No.:BCX1590
CAS No.:297749-29-2
- Wuweizidilactone A
Catalog No.:BCX1591
CAS No.:945610-99-1
- Daphneticin
Catalog No.:BCX1592
CAS No.:83327-22-4
- Telephiose C
Catalog No.:BCX1593
CAS No.:297749-31-6
- Vinaginsenoside R7
Catalog No.:BCX1594
CAS No.:156009-83-5
- Oleaside B
Catalog No.:BCX1595
CAS No.:71699-08-6
- Arisanlactone D
Catalog No.:BCX1596
CAS No.:1234186-01-6
- 14-Formyldihydrorutaecarpine
Catalog No.:BCX1597
CAS No.:68353-23-1
- Apigenin-7-O-glucuronide-6'-ethyl ester
Catalog No.:BCX1598
CAS No.:62268-42-2
Revealing the active ingredients and mechanism of P. sibiricumm in non-small-cell lung cancer based on UPLC-Q-TOF-MS/MS, network pharmacology, and molecular docking.[Pubmed:38617965]
Heliyon. 2024 Apr 3;10(7):e29166.
The alcohol extraction of P. sibiricum has exhibited significant inhibitory effects on the production of free radicals and the proliferation of non-small-cell lung carcinoma (NSCLC) A549 cells. Despite the diverse components found in alcohol extraction of P. sibiricum and its multiple targets, the active components and associated targets remain largely unidentified. Hence, there is a need for additional investigation into the pharmacodynamic elements and mechanisms of action. This study aimed to analyze and identify the components responsible for the anti-tumor activity of alcohol extraction from P. sibiricum using UPLC-Q-TOF-MS/MS for the first time. Subsequently, the targets of the active components were predicted using the SwissTargetPrediction database, whereas the targets for NSCLC were sourced from the Online Mendelian Inheritance in Man database (OMIM) and the GeneCards database. Next, the targets of chemical composition were integrated with disease targets via Venny online. GO and KEGG pathway enrichment analyses were performed utilizing DAVID. Subsequently, a network analysis of "components-targets-pathways" was established using Cytoscape 3.8.2 and assessed with the "network analyzer" plug-in. Molecular docking was conducted utilizing Autodock 1.5.6. The study aimed to examine the anti-proliferative impacts and underlying mechanisms of alcohol extraction from P. sibiricum on NSCLC through in vivo and in vitro investigations utilizing an animal model of transplanted tumor, CCK8 assay, cell scratch test, RT-qPCR, and western blotting. The study unveiled that 17 active components extracted from P. sibiricum alcohol demonstrated anti-non-small cell lung cancer (NSCLC) effects through the modulation of 191 targets and various significant signaling pathways. These pathways include Endocrine resistance, PI3K/AKT, Chemical carcinogenesis-receptor activation, Proteoglycans in cancer, EGFR tyrosine kinase inhibitor resistance, AMPK signaling pathway, and other related signaling pathways. Network analysis and molecular docking results indicated that specific compounds such as (25S)-26-O-(beta-d-glucopyranosyl)-furost-5-en3beta,22alpha,26-triol3-O-beta-d-glucopyranosyl-(1-->2)-beta-d-glucopyranosyl-(1-->4)-beta-d-glucopyranoside, Timosaponin H1, Deapi-platycodin D3, (3R)-5,7-dihydroxy-6,8-dimethyl-3-(4'-hydroxybenzyl)-chroman-4-one, Disporopsin, Funkioside F, Kingianoside E, Parisyunnanoside H, and Sibiricoside B primarily targeted 17 key proteins (BCL2, EGFR, ESR1, ESR2, GRB2, IGF1R, JUN, MAP2K1, MAPK14, MAPK8, MDM2, MMP9, mTOR, PIK3CA, RAF1, RPS6KB1, and SRC) collectively. In conclusion, the alcohol extraction of P. sibiricum demonstrated inhibitory effects on cell proliferation, induction of apoptosis, and inhibition of metastasis through various pathways.
Qualitative and quantitative studies on two commercial specifications of Polygonatum odoratum.[Pubmed:36909715]
Front Chem. 2023 Feb 23;11:1146153.
The rhizoma of Polygonatum odoratum (PO) is used to treat yin injuries of the lung and stomach in traditional Chinese medicine. The chemical constituents of this herb are steroidal saponins, homoisoflavanones, and alkaloids. Xiangyuzhu (XPO) and Guanyuzhu (GPO) are available in the market as two specifications of the commodity. Nonetheless, systematic research on the identification and comparison of chemical constituents of these two commercial specifications is yet lacking. Herein, an integrated method combing ultra-high-performance liquid chromatography-quadruple time-of-flight mass spectrometry (UHPLC-Q-TOF/MS) with ultra-high-performance liquid chromatography-charged aerosol detection (UHPLC-CAD) was employed for the comprehensively qualitative and quantitative analyses of PO. A total of 62 compounds were identified by UHPLC-Q-TOF/MS, among which 13 potential chemical markers were screened out to distinguish two commercial specifications. Subsequently, the absolute determination method for polygodoraside G, polygonatumoside F, and Timosaponin H1 was established and validated by UHPLC-CAD. The contents of the three compounds were 13.33-236.24 mug/g, 50.55-545.04 mug/g, and 13.34-407.83 mug/g, respectively. Furthermore, the ratio of Timosaponin H1/polygodoraside G could be applied to differentiate the two specifications. Samples with a ratio <2 are considered XPO and >5 are considered GPO. Therefore, the above results provide a valuable means for the quality control of PO.
Development and validation of liquid chromatography-tandem mass spectrometry method for simultaneous determination of six steroidal saponins in rat plasma and its application to a pharmacokinetics study.[Pubmed:25617741]
Steroids. 2015 Apr;96:21-9.
A specific and reliable liquid chromatography-electrospray ionization-tandem mass spectrometry method was developed for the simultaneous determination of Timosaponin H1 (TH1), timosaponin E1 (TE1), timosaponin E (TE), timosaponin B-II (TB-II), timosaponin B-III (TB-III) and anemarrhenasaponin I (AS-I) in rat plasma. After addition of internal standard (IS) ginsenoside Rh1, plasma samples were pretreated by protein precipitation with acetonitrile. Chromatographic separation was performed on a reverse phase ACQUITY BEH C18 column (100mmx2.1mm i.d., 1.7mum) using a gradient mobile phase system of acetonitrile-water containing 0.05% formic acid and 5mM ammonium formate. The triple quadruple mass spectrometer was set in negative electrospray ionization mode and multiple reaction monitoring (MRM) was used for six steroidal saponins quantification. The precursors to produce ion transitions monitored for TH1, TE1, TE, TB-II, TB-III, AS-I and IS were m/z 1211.5>1079.6, 935.5>773.4, 935.4>773.5, 919.6>757.4, 901.5>739.3, 757.4>595.3 and 637.3>475.3, respectively. The method validation was conducted over the curve range of 0.5-400ng/mL for the six saponins. The intra- and inter-day precisions (RSD%) were less than 9.4% and the average extraction recoveries ranged from 82.5% to 97.8% for each analyte. Six steroidal saponins were proved to be stable during sample storage, preparation and analytical procedures. The validated method was successfully applied for the first time to determine the concentrations of six main steroidal saponins in incurred rat plasma samples, after intragastric administration of the extract of Anemarrhena asphodeloides Bge. for a rat pharmacokinetic study.
Two new furostanol saponins from Aspidistra typica.[Pubmed:23679565]
J Asian Nat Prod Res. 2013;15(5):525-31.
Two new furostanol saponins (1 and 2), along with one known saponin (3), were obtained from the rhizomes of Aspidistra typica Baill. Their structures were elucidated as (25R)-26-O-beta-d-glucopyranosyl-furost-5-ene-12-one-3beta,22alpha,26-triol-3-O-beta-d-glucopyranosyl-(1 --> 2)-[beta-d-xylopyranosyl-(1 --> 3)]-beta-d-glucopyranosyl-(1 --> 4)-beta-d-galactopyranoside (1, typaspidoside A), (25S)-26-O-beta-d-glucopyranosyl-furost-5-ene-12-one-3beta,22alpha,26-triol-3-O-beta-d-glucopyranosyl-(1 --> 2)-[beta-d-xylopyranosyl-(1 --> 3)]-beta-d-glucopyranosyl-(1 --> 4)-beta-d-galactopyranoside (2, 25S-typaspidoside A), and Timosaponin H1 (3), based on the integrative spectroscopic analysis of 1D- and 2D-NMR experiments, ESI-MS data and chemical evidence. The investigation on the chemical components of this plant is reported for the first time.


