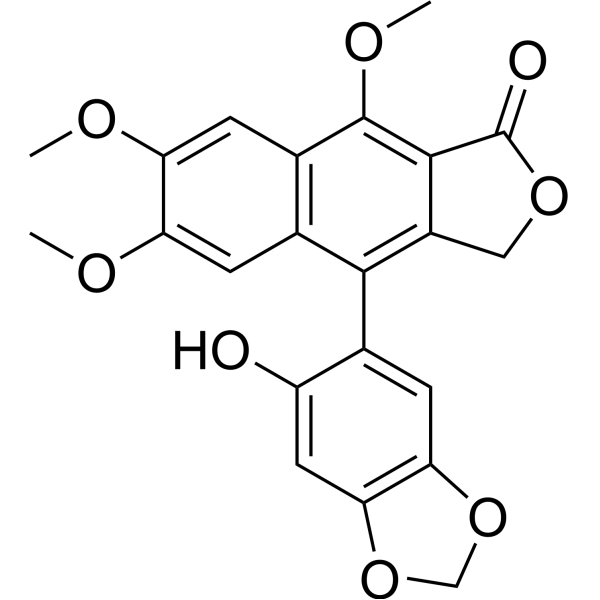
6'-Hydroxyjusticidin CCAS# 904328-26-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 904328-26-3 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C22H18O8 | M.Wt | 410.37 |
| Type of Compound | Lignanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

6'-Hydroxyjusticidin C Dilution Calculator

6'-Hydroxyjusticidin C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4368 mL | 12.1841 mL | 24.3683 mL | 48.7365 mL | 60.9206 mL |
| 5 mM | 0.4874 mL | 2.4368 mL | 4.8737 mL | 9.7473 mL | 12.1841 mL |
| 10 mM | 0.2437 mL | 1.2184 mL | 2.4368 mL | 4.8737 mL | 6.0921 mL |
| 50 mM | 0.0487 mL | 0.2437 mL | 0.4874 mL | 0.9747 mL | 1.2184 mL |
| 100 mM | 0.0244 mL | 0.1218 mL | 0.2437 mL | 0.4874 mL | 0.6092 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Malabaricone B
Catalog No.:BCX1677
CAS No.:63335-24-0
- Macrostemonoside I
Catalog No.:BCX1676
CAS No.:162413-63-0
- Peimisine 3-O-β-D-glucopyranoside
Catalog No.:BCX1675
CAS No.:1407161-78-7
- Cararosinol A
Catalog No.:BCX1674
CAS No.:651733-57-2
- Lupulone C
Catalog No.:BCX1673
CAS No.:613683-50-4
- Garcinielliptone HD
Catalog No.:BCX1672
CAS No.:1008376-90-6
- Anhuienoside B
Catalog No.:BCX1671
CAS No.:1233510-75-2
- 19-epi-Scholaricine
Catalog No.:BCX1670
CAS No.:132923-06-9
- Odoratisol B
Catalog No.:BCX1669
CAS No.:891182-94-8
- Uvariol
Catalog No.:BCX1668
CAS No.:56362-97-1
- Copteroside G
Catalog No.:BCX1667
CAS No.:86438-31-5
- Inonotusol F
Catalog No.:BCX1666
CAS No.:1534433-74-3
- α-Thevetin B
Catalog No.:BCX1679
CAS No.:144300-21-0
- Cannogenol 3-O-β-gentiobiosyl-(1→4)-α-L-thevetoside
Catalog No.:BCX1680
CAS No.:1841524-78-4
- 2'- O -Acetylthevetin A
Catalog No.:BCX1681
CAS No.:1356494-03-5
- (7’S)-N-trans-Feruloylnormetanephrine
Catalog No.:BCX1682
CAS No.:2705170-78-9
- Rubrofusarin
Catalog No.:BCX1683
CAS No.:3567-00-8
- (1β,2β,3β,4β,5β,7α,22α)-27-(β-D-Glucopyranosyloxy)-1,2,3,4,5,7,22-heptahydroxyfurost-25-en-6-one
Catalog No.:BCX1684
CAS No.:1013405-26-9
- 2'- O -Acetylthevetin B
Catalog No.:BCX1685
CAS No.:82145-55-9
- Paclobutrazol
Catalog No.:BCX1686
CAS No.:76738-62-0
- Flaccidoside III
Catalog No.:BCX1687
CAS No.:140400-67-5
- Protoapigenone
Catalog No.:BCX1688
CAS No.:862884-32-0
- Glycoside St-J
Catalog No.:BCX1689
CAS No.:203513-88-6
- 2-Mialine
Catalog No.:BCX1690
CAS No.:634-97-9
A Strategy for Preparative Separation of 10 Lignans from Justicia procumbens L. by High-Speed Counter-Current Chromatography.[Pubmed:29168751]
Molecules. 2017 Nov 23;22(12):2024.
Ten compounds, including three lignan glycosides and seven lignans, were purified from Justicia procumbens L. in 8 h using an efficient strategy based on high-speed counter-current chromatography (HSCCC). The two-phase solvent system composed of petroleum-ethyl acetate-methanol-H(2)O (1:0.7:1:0.7, v/v) was firstly employed to separate the crude extract (320 mg), from which 19.3 mg of justicidin B (f), 10.8 mg of justicidin A (g), 13.9 mg of 6'-hydroxyjusticidin C (h), 7.7 mg of justicidin E (i), 6.3 mg of lignan J(1) (j) were obtained with 91.3 mg of enriched mixture of compounds a-e. The enriched mixture (91.3 mg) was further separated using the solvent system consisting of petroleum-ethyl acetate-methanol-H(2)O (3:3.8:3:3.8, v/v), yielding 12.1 mg of procumbenoside E (a); 7.6 mg of diphyllin-1-O-beta-d-apiofuranoside (b); 7.4 mg of diphyllin (c); 8.3 mg of 6'-hydroxy justicidin B (d); and 7.9 mg of diphyllin acetyl apioside (e). The purities of the 10 components were all above 94%, and their structures were identified by NMR and ESI-MS spectra. The results demonstrated that the strategy based on HSCCC for the separation of lignans and their glycosides was efficient and rapid.
Preparative isolation and purification of lignans from Justicia procumbens using high-speed counter-current chromatography in stepwise elution mode.[Pubmed:25903362]
Molecules. 2015 Apr 20;20(4):7048-58.
Lignans, which are recognized as main constituents in Justicia procumbens, have attracted considerable attention due to their pharmacological activities, including antitumor, anti-hepatitic, cytotoxic, anti-microbial, and anti-virus properties. Preparative high-speed counter-current chromatography (HSCCC) was successfully applied to the isolation and purification of four lignans (justicidin B (1), justicidin A (2), 6'-hydroxyjusticidin C (3) and lignan J1 (4)) from J. procumbens using stepwise elution with a pair of two-phase solvent systems composed of n-hexane-ethyl acetate-methanol-water at (1.3:1:1.3:1, v/v) and (2.5:1:2.5:1, v/v). The preparative HSCCC separation was performed on 300 mg of crude sample yielding compounds 1 (19.7 mg), 2 (9.86 mg), 3 (11.26 mg), and 4 (2.54 mg) in a one-step separation, with purities over 95% as determined by HPLC. The structures of these compounds were identified by MS, 1H-NMR and 13C-NMR. This is the first report on the application of HSCCC to the efficient separation of lignans from J. procumbens.


