ProtoapigenoneCAS# 862884-32-0 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 862884-32-0 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
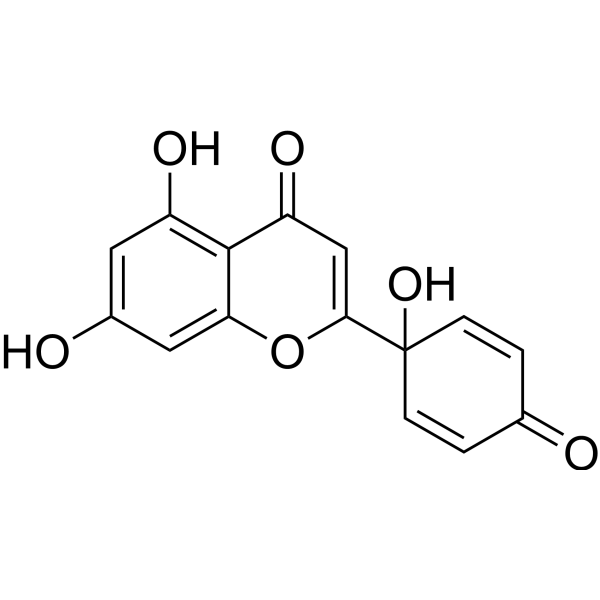
| Formula | C15H10O6 | M.Wt | 286.24 |
| Type of Compound | Flavones/Flavanones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Protoapigenone Dilution Calculator

Protoapigenone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4936 mL | 17.4679 mL | 34.9357 mL | 69.8714 mL | 87.3393 mL |
| 5 mM | 0.6987 mL | 3.4936 mL | 6.9871 mL | 13.9743 mL | 17.4679 mL |
| 10 mM | 0.3494 mL | 1.7468 mL | 3.4936 mL | 6.9871 mL | 8.7339 mL |
| 50 mM | 0.0699 mL | 0.3494 mL | 0.6987 mL | 1.3974 mL | 1.7468 mL |
| 100 mM | 0.0349 mL | 0.1747 mL | 0.3494 mL | 0.6987 mL | 0.8734 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Flaccidoside III
Catalog No.:BCX1687
CAS No.:140400-67-5
- Paclobutrazol
Catalog No.:BCX1686
CAS No.:76738-62-0
- 2'- O -Acetylthevetin B
Catalog No.:BCX1685
CAS No.:82145-55-9
- (1β,2β,3β,4β,5β,7α,22α)-27-(β-D-Glucopyranosyloxy)-1,2,3,4,5,7,22-heptahydroxyfurost-25-en-6-one
Catalog No.:BCX1684
CAS No.:1013405-26-9
- Rubrofusarin
Catalog No.:BCX1683
CAS No.:3567-00-8
- (7’S)-N-trans-Feruloylnormetanephrine
Catalog No.:BCX1682
CAS No.:2705170-78-9
- 2'- O -Acetylthevetin A
Catalog No.:BCX1681
CAS No.:1356494-03-5
- Cannogenol 3-O-β-gentiobiosyl-(1→4)-α-L-thevetoside
Catalog No.:BCX1680
CAS No.:1841524-78-4
- α-Thevetin B
Catalog No.:BCX1679
CAS No.:144300-21-0
- 6'-Hydroxyjusticidin C
Catalog No.:BCX1678
CAS No.:904328-26-3
- Malabaricone B
Catalog No.:BCX1677
CAS No.:63335-24-0
- Macrostemonoside I
Catalog No.:BCX1676
CAS No.:162413-63-0
- Glycoside St-J
Catalog No.:BCX1689
CAS No.:203513-88-6
- 2-Mialine
Catalog No.:BCX1690
CAS No.:634-97-9
- Bruceantarin
Catalog No.:BCX1691
CAS No.:41451-76-7
- (+)-(3’S,4’R)-3’-acetyl-4’-isobutyrylkhellactone
Catalog No.:BCX1692
CAS No.:1562996-11-5
- Arborescosidic acid
Catalog No.:BCX1693
CAS No.:325798-51-4
- Apigenin-7-O-diglucuronoside
Catalog No.:BCX1694
CAS No.:119738-57-7
- Complanatin II
Catalog No.:BCX1695
CAS No.:142449-94-3
- Ladanetin-6-O-β-(6''-O- acetyl)glucoside
Catalog No.:BCX1696
CAS No.:1256959-27-9
- Cirsiliol 4'-glucoside
Catalog No.:BCX1697
CAS No.:41087-98-3
- Pedaliin 6''-acetate
Catalog No.:BCX1698
CAS No.:160789-41-3
- Ladanetin-6-O-β-D-glucopyranoside
Catalog No.:BCX1699
CAS No.:89647-63-2
- Homoveratric acid
Catalog No.:BCX1700
CAS No.:93-40-3
A novel protoapigenone analog RY10-4 induces apoptosis of breast cancer cells by exacerbating mitochondrial Ca(2+) influx through mitochondrial calcium uniporter.[Pubmed:34717963]
Toxicol Appl Pharmacol. 2021 Dec 15;433:115776.
RY10-4, a novel Protoapigenone analog with a specific nonaromatic B-ring, displayed enhanced cytotoxicity in various tumor cells, especially for breast cancer cells, but the underlying mechanism remains unclear. In the present study, we confirmed the pro-apoptotic effect of RY10-4 on breast cancer cells. Furthermore, mitochondrial calcium uniporter (MCU) was proved to be up-regulated in RY10-4-treated MDA-MB-231 cells, which resulted in the overload of mitochondrial calcium ([Ca(2+)]m) and subsequently disrupted mitochondrial functions (characterized by mitochondrial reactive oxygen species (mtROS) accumulation, membrane potential (DeltaPsim) depolarization and permeability transition pore (mPTP) opening). And finally, the mitochondrial apoptosis was activated by the release of cytochrome C. Interestingly, knockdown of MCU attenuated the overload of [Ca(2+)]m and blocked the apoptosis of MDA-MB-231 cells induced by RY10-4, which was consistent with the in vivo results. Taken together, this study proved that RY10-4 could induce apoptosis of breast cancer cells by elevating [Ca(2+)]m through MCU. Our work contributed previously unknown insights into the mechanisms involving in the clinical efficacy of RY10-4 on breast cancer cells, which also advanced calcium homeostasis as a potential target for chemotherapeutic drugs.
Identification of Potential Inhibitors against Epstein-Barr Virus Nuclear Antigen 1 (EBNA1): An Insight from Docking and Molecular Dynamic Simulations.[Pubmed:34340305]
ACS Chem Neurosci. 2021 Aug 18;12(16):3060-3072.
Epstein-Barr virus (EBV), a known tumorigenic virus, is associated with various neuropathies, including multiple sclerosis (MS). However, there is no anti-EBV FDA-approved drug available in the market. Our study targeted EBV protein EBV nuclear antigen 1 (EBNA1), crucial in virus replication and expressed in all the stages of viral latencies. This dimeric protein binds to an 18 bp palindromic DNA sequence and initiates the process of viral replication. We chose phytochemicals and FDA-approved MS drugs based on literature survey followed by their evaluation efficacies as anti-EBNA1 molecules. Molecular docking revealed FDA drugs ozanimod, siponimod, teriflunomide, and phytochemicals; emodin; Protoapigenone; and EGCG bound to EBNA1 with high affinities. ADMET and Lipinski's rule analysis of the phytochemicals predicted favorable druggability. We supported our assessments of pocket druggability with molecular dynamics simulations and binding affinity predictions by the molecular mechanics generalized Born surface area (MM/GBSA) method. Our results establish a stable binding for siponimod and ozanimod with EBNA1 mainly via van der Waals interactions. We identified hot spot residues like I481', K477', L582', and K586' in the binding of ligands. In particular, K477' at the amino terminal of EBNA1 is known to establish interaction with two bases at the major groove of the DNA. Siponimod bound to EBNA1 engaging K477', thus plausibly making it unavailable for DNA interaction. Computational alanine scanning further supported the significant roles of K477', I481', and K586' in the binding of ligands with EBNA1. Conclusively, the compounds showed promising results to be used against EBNA1.
Protoflavones in melanoma therapy: Prooxidant and pro-senescence effect of protoapigenone and its synthetic alkyl derivative in A375 cells.[Pubmed:32931795]
Life Sci. 2020 Nov 1;260:118419.
AIMS: In our study, the anticancer effects of a semisynthetic p-quinol, Protoapigenone 1'-O-butyl ether (PABut), were tested in human melanoma A375 cells also in comparison with natural congener, Protoapigenone (PA). MAIN METHODS: The cytotoxic effect of PABut and PA was determined using MTT assay. Flow cytometry analysis was used to evaluate the influence of the compounds tested on ROS generation and cell cycle distribution in A375 cells. Moreover, apoptosis was evaluated by AO/EB dual staining as well as by flow cytometry. Markers of senescence were quantified by spectrofluorimetry and by Western blot analysis. KEY FINDINGS: Both PABut and PA showed significant cytotoxicity against melanoma A375 cells at sub-micromolar concentrations. Both protoflavones induced comparable cell cycle arrest in G2/M phase. However, a more profound upregulation of intracellular ROS levels was found following PABut treatment. An increased apoptosis in the cells following 48 h treatment with both protoflavones tested was also confirmed. Both compounds tested remarkably upregulated p21 protein levels in A375 cells. Unlike PA, PABut significantly decreased protein levels of NAD(+)-dependent deacetylase SirT1 and beta-actin accompanied by mild significant upregulation of mitochondrial SOD2 and senescence markers, p16 protein and SA-beta-Gal activity. However, a significant upregulation of p53 only following PA treatment was found. SIGNIFICANCE: These results suggest that PABut and PA confer high chemotherapeutic potential in melanoma cells and are suitable for further testing. Furthermore, modification of Protoapigenone with 1'-O-butyl ether moiety can be associated with improved senescence-inducing effect and, thus, enhanced chemotherapeutic potency of PABut compared to the unmodified natural protoflavone.
The protoapigenone analog WYC0209 targets CD133+ cells: A potential adjuvant agent against cancer stem cells in urothelial cancer therapy.[Pubmed:32673656]
Toxicol Appl Pharmacol. 2020 Sep 1;402:115129.
Urothelial carcinoma (UC) is one of the highest incidence cancers that rank the fourth commonly diagnosed tumors worldwide. The unresectable lesions that are resistant to therapeutic interventions is the major cause leading to death. Previous studies had shown that the resistance and metastatic consequence may arise from cancer stem-like cells population. The phytochemical flavonoids have promised bioactivity and potent anti-carcinogenic effects, and trap great attentions for cancer chemoprevention and/or adjuvant chemotherapy. However, the mechanisms of flavonoids on cancer stemness is still obscured. In this study, we analyzed the biofunctional effects of as-prepared flavonoid derivative-WYC0209 on T24, BFTC905 and BFTC909 human UC cell lines. Our results demonstrated that WYC0209 significantly induced anti-cell viability on UC cells through decreased Akt/NFkB signaling. Moreover, WYC0209 enhanced the cell apoptosis through activated the caspase-3 activity and inactivated Bcl-xL expression. Interestingly, WYC0209 dramatically inhibited the cancer stem cells (CSCs) traits, including attenuation of side population and tumorsphere formation in which were through declined EMT-CSCs markers including MDR1, ABCG2 and BMI-1. We further validated the effects of WYC0209 on several CSC surface markers including CD133, CD44, SOX-2 and Nanog. Our results showed that WYC0209 markedly inhibited CD133 expressions in both transcriptional and translational levels. High expression levels of CD133 was also demonstrated in human upper tract UC specimens. In summary, our study showed that WYC0209 may potentially as an adjuvant agent to against CD133-driven UC CSCs and provide a beneficial strategy to against UC cancer therapeutics resistant.
Protoflavone-Chalcone Hybrids Exhibit Enhanced Antitumor Action through Modulating Redox Balance, Depolarizing the Mitochondrial Membrane, and Inhibiting ATR-Dependent Signaling.[Pubmed:32545536]
Antioxidants (Basel). 2020 Jun 12;9(6):519.
Hybrid compounds combine fragments with complementary targets to achieve a common pharmacological goal. This approach represents an increasingly popular strategy for drug discovery. In this work, we aimed to design antitumor hybrid compounds based on an inhibitor of ataxia-telangiectasia and Rad3-related protein (ATR)-dependent signaling, Protoapigenone, and a pro-oxidant ferrocene or chalcone fragment. Four new triazole-coupled hybrids were prepared. The compounds were cytotoxic against human breast cancer cell lines in vitro, showing IC(50) values in the sub-micromolar range. The nature of interactions between relevant fragments of the hybrids was evaluated by the Chou-Talalay method. Experimental combination treatment with the fragments showed additive effects or slight/moderate synergism, while strong synergism was observed when the fragments were virtually combined into their hybrids, suggesting a relevant pharmacological benefit of the coupling. All hybrids were strong inhibitors of the ATR-mediated activation of Chk1, and they interfered with the redox balance of the cells leading to mitochondrial membrane depolarization. Additionally, they induced late apoptosis and primary necrosis in MDA-MB-231 and MCF-7 breast cancer cells, respectively. Our results demonstrate that coupling the ATR-dependent signaling inhibitor protoflavone with a pro-oxidant chalcone dramatically increases the antitumor activity compared with either fragment alone. Such compounds may offer an attractive novel strategy for the treatment of various cancers.
Less Cytotoxic Protoflavones as Antiviral Agents: Protoapigenone 1'-O-isopropyl ether Shows Improved Selectivity Against the Epstein-Barr Virus Lytic Cycle.[Pubmed:31842358]
Int J Mol Sci. 2019 Dec 12;20(24):6269.
Protoflavones, a rare group of natural flavonoids with a non-aromatic B-ring, are best known for their antitumor properties. The protoflavone B-ring is a versatile moiety that might be explored for various pharmacological purposes, but the common cytotoxicity of these compounds is a limitation to such efforts. Protoapigenone was previously found to be active against the lytic cycle of Epstein-Barr virus (EBV). Further, the 5-hydroxyflavone moiety is a known pharmacophore against HIV-integrase. The aim of this work was to prepare a series of less cytotoxic protoflavone analogs and study their antiviral activity against HIV and EBV. Twenty-seven compounds, including 18 new derivatives, were prepared from apigenin through oxidative de-aromatization and subsequent continuous-flow hydrogenation, deuteration, and/or 4'-oxime formation. One compound was active against HIV at the micromolar range, and three compounds showed significant activity against the EBV lytic cycle at the medium-low nanomolar range. Among these derivatives, Protoapigenone 1'-O-isopropyl ether (6) was identified as a promising lead that had a 73-times selectivity of antiviral over cytotoxic activity, which exceeds the selectivity of Protoapigenone by 2.4-times. Our results open new opportunities for designing novel potent and safe anti-EBV agents that are based on the natural protoflavone moiety.
The antagonism between apigenin and protoapigenone to the PDK-1 target in Macrothelypteris torresiana.[Pubmed:30731149]
Fitoterapia. 2019 Apr;134:14-22.
Apigenin and Protoapigenone that both have the activities against various cancer cell lines co-exist in Macrothelypteris torresiana, while the extracts of M. torresiana couldn't achieve the fine anti-tumor effects for the existence of potent anti-tumor compounds. This study disclosed an antagonism between the two compounds on the protein level to elucidate the paradox. First, the study established the fingerprint for M. torresiana extract. The following anti-proliferation assay verified that the antagonism occurs between Protoapigenone and apigenin. And then Western blot and qt-PCR were applied to evaluate the expression and transcription level of the Akt phosphorylation related targets to validate the antagonism at the protein level. Moreover, CETSA further validated the binding of PDK-1 with apigenin and Protoapigenone, as well as the antagonism between the two compounds. Finally, the compound-protein complexes predicted by SYBYL-X gave the visual results for the antagonism. The results demonstrated that: Due to the structural similarity and close binding coefficients to the identical targets, when the cells were treated with apigenin and Protoapigenone simultaneously, the Akt phosphorylation inhibition induced by Protoapigenone would attenuate significantly. The antagonism disclosed in this paper could be a new explanation for the unsatisfied efficacy of M. torresiana extract.
Synthesis of Nontoxic Protoflavone Derivatives through Selective Continuous-Flow Hydrogenation of the Flavonoid B-Ring.[Pubmed:31957309]
Chempluschem. 2018 Feb;83(2):72-76.
Protoflavones are unique natural flavonoids with a non-aromatic B-ring, known for their potent antitumor properties. However, their cytotoxicity represents a strong limitation in the further exploration of their pharmacological potential. In the current study, we sought to selectively saturate the p-quinol B-ring of Protoapigenone and that of its 1'-O-butyl ether, in order to obtain non-toxic protoflavone analogues expressing the dihydro- or tetrahydroprotoflavone structure also occurring in nature. The benefits of a strictly controlled continuous-flow environment in combination with on-demand electrolytic H(2) gas generation were exploited to suppress undesired side reactions and to safely and selectively yield the desired substances. The obtained tetrahydroprotoflavones were free of the cytotoxicity of their parent compounds, and, even though tetrahydroProtoapigenone 1-O-butyl ether showed a weak inhibition of DNA damage response through Chk1, neither compounds influenced the cytotoxicity of doxorubicin either.
Synthesis and SAR Study of Anticancer Protoflavone Derivatives: Investigation of Cytotoxicity and Interaction with ABCB1 and ABCG2 Multidrug Efflux Transporters.[Pubmed:28436164]
ChemMedChem. 2017 Jun 7;12(11):850-859.
There is a constant need for new therapies against multidrug-resistant (MDR) cancer. Natural compounds are a promising source of novel anticancer agents. We recently showed that protoflavones display activity in MDR cancer cell lines that overexpress the P-glycoprotein (P-gp) drug efflux pump. In this study, 52 protoflavones, including 22 new derivatives, were synthesized and tested against a panel of drug-sensitive parental cells and their MDR derivatives obtained by transfection with the human ABCB1 or ABCG2 genes, or by adaptation to chemotherapeutics. With the exception of Protoapigenone, identified as a weak ABCG2 substrate, all protoflavones bypass resistance conferred by these two transporters. The majority of the compounds were found to exhibit mild to strong (up to 13-fold) selectivity against the MCF-7(Dox) and KB-V1 cell lines, but not to transfected MDR cells engineered to overexpress the MDR transporters. Our results suggest that protoflavones can overcome MDR cancer by evading P-gp-mediated efflux.
RY10-4 Inhibits the Proliferation of Human Hepatocellular Cancer HepG2 Cells by Inducing Apoptosis In Vitro and In Vivo.[Pubmed:26974964]
PLoS One. 2016 Mar 14;11(3):e0151679.
This study aimed to investigate the anti-tumor activity of RY10-4, a small molecular that was designed and synthesized based on the structure of Protoapigenone. A previous screening study showed that RY10-4 possessed anti-proliferative effects against HepG2 human hepatocellular carcinoma cells. However, the full range of RY10-4 anti-cancer effects on liver tumors and the underlying mechanisms have not been identified. Herein, employing flow cytometry, and Western blot analysis, we demonstrate that RY10-4 can induce cell cycle arrest, intracellular reactive oxygen species (ROS) production and apoptosis in HepG2 cells. In HepG2 cell xenograft tumor model, RY10-4 significantly inhibited the growth of tumors and induced apoptosis in tumor cells, with little side effects. Moreover, RY10-4 caused the suppression of STAT3 activation, which may be involved the apoptosis induction. In addition, RY10-4 inhibited the proliferation of Hep3B and HuH-7 human hepatocellular carcinoma cells in a concentration-dependent manner. Taken together, our results suggest that RY10-4 has a great potential to develop as chemotherapeutic agent for liver cancer.
Combination therapy of RY10-4 with the gamma-secretase inhibitor DAPT shows promise in treating HER2-amplified breast cancer.[Pubmed:26716652]
Oncotarget. 2016 Jan 26;7(4):4142-54.
RY10-4, a novel Protoapigenone analog, shows potent cytotoxicity against human breast cancer cells. However, breast cancer cell lines overexpressing human epidermal growth factor receptor 2 (HER2), SKBR3 and BT474, showed less sensitivity to RY10-4 when compared to breast cancer cells lines expressing lower levels of HER2, such as MDA-MB-231 and MCF-7 cells. This was associated with aberrant hyperactivity in Notch signaling in cells treated with RY10-4, since treatment with RY10-4 causes an increase in Notch activity by 2-to3.5-fold in SKBR3 and BT474 cell lines. The increase in activity was abrogated with a gamma-secretase inhibitor, DAPT, or with Notch1 small-interfering RNA (si-Notch1). Cell proliferation was inhibited more effectively by RY10-4 plus DAPT or si-Notch1 than either agent alone. RY10-4 plus DAPT increases apoptosis in both HER2-overexpressing cell lines by two-fold compared to RY10-4 alone, while DAPT alone has no significant effects on apoptosis. In addition, we previously found RY10-4 could inhibit tumor growth through the PI3K/AKT pathway. Here we report that the combination of RY10-4 and DAPT exhibit additive suppression on AKT phosphorylation, contributing to the anti-cancer effects. In an animal model, this combination therapy inhibits the growth of SKBR3 tumor xenografts in nude mice to a greater extent than treatment with either reagent alone. These results indicate that the aberrant activation of Notch signaling impedes the inhibitory effect of RY10-4 on HER2-amplified cell proliferation. Furthermore, these adverse effects can be prevented by treatment combining RY10-4 with a Notch pathway inhibitor.
Preparation of magnetic molecularly imprinted polymers for selective isolation and determination of kaempferol and protoapigenone in Macrothelypteris torresiana.[Pubmed:25480580]
J Huazhong Univ Sci Technolog Med Sci. 2014 Dec;34(6):845-855.
Novel uniform-sized magnetic molecularly imprinted polymers (MMIPs) were synthesized for selective recognition of active antitumor ingredients of kaempferol (KMF) and Protoapigenone (PA) in Macrothelypteris torresiana (M. torresiana) by surface molecular imprinting technique in this study. Super paramagnetic core-shell nanoparticles (gamma-MPS-SiO2@Fe3O4) were used as seeds, KMF as template molecule, acrylamide (AM) as functional monomer, and N, N'-methylene bisacrylamide (BisAM) as cross-linker. The prepared MMIPs were characterized by X-ray diffraction (XRD), Fourier transform infrared spectrum (FTIR), transmission electron microscopy (TEM) and thermo-gravimetric analysis (TGA), respectively. The recognition capacity of MMIPs was 2.436 times of non-imprinted polymers. The adsorption results based on kinetics and isotherm analysis were in accordance with the pseudo-second-order model (R (2)=0.9980) and the Langmuir adsorption model (R (2)=0.9944). The value of E (6.742 kJ/mol) calculated from the Dubinin-Radushkevich isotherm model suggested that the physical adsorption via hydrogen-bonding might be predominant. The Scatchard plot showed a single line (R (2)=0.9172) and demonstrated the homogeneous recognition sites on MMIPs for KMF. The magnetic solid phase extraction (MSPE) based on MMIPs as sorbent was established for fast and selective enrichment of KMF and its structural analogue PA from the crude extract of M. torresiana and then KMF and PA were detected by HPLC-UV. The established method showed good performance and satisfactory results for real sample analysis. It also showed the feasibility of MMIPs for selective recognition of active structural analogues from complex herbal extracts.
A novel compound RY10-4 downregulates P-glycoprotein expression and reverses multidrug-resistant phenotype in human breast cancer MCF-7/ADR cells.[Pubmed:25455158]
Biomed Pharmacother. 2014 Oct;68(8):1049-56.
P-glycoprotein (P-gp), an important efflux transporter, is encoded by the MDR1 class of genes and is a major element of the multidrug resistance (MDR) phenomenon in breast cancers. The most common approved cause of MDR in cancer tissues is the over-expression of P-gp. At present, a novel potent anti-tumor compound RY10-4 has been synthesized by our team, which has a similar structure close to Protoapigenone. We chose MCF-7/ADR cells, an adriamycin (ADR) - selected human breast tumor cell line with the MDR phenotype, to study the anticancer features of this novel compound in our experiments. In cytotoxicity and apoptosis tests, it was shown that RY10-4 significantly inhibited cell growth, induced apoptosis, potentiated ADR cytotoxicity and restored chemotherapy sensitivity in the MDR cancer cells. Furthermore, our results suggested that RY10-4 reversed MDR partially by down-regulation of P-gp and MDR1 expressions in the MCF-7/ADR cell line. Besides, it is seen that RY10-4 could reduce the intracellular ATP level. Our studies give the theoretical basis for the possible clinical applications of RY10-4 alone or in combination with other chemotherapeutic drugs in the treatment of MDR tumors.
RY10-4 suppressed metastasis of MDA-MB-231 by stabilizing ECM and E-cadherin.[Pubmed:24721328]
Biomed Pharmacother. 2014 May;68(4):439-45.
In the article, we investigated the anti-metastasis mechanism of RY10-4, an anti-tumor compound derived from Protoapigenone, in breast tumor cells MB-MDA-231. The analog of Protoapigenone with an unaromatic B-ring was verified to suppress the proliferation of several tumor cells by previous research that also showed that several tumor progression such as inducing apoptosis and anti-angiogenesis could be acted on by RY10-4. In the article, we investigated the mechanism about how RY10-4 suppressed the invasion of MDA-MB-231. Firstly, the transwells assays with and without matrigel were adapted to evaluate the anti-metastasis and anti-invasion activity. Much research had demonstrated that the ECM and E-cadherin/beta-catenin complex play an important role in cell adhesion and the formation of the cell skeleton, and as we knew the abnormal and absent expression of ECM and E-cadherin/beta-catenin complex are found in many malignant cells. The result demonstrated that the amount and distribution of E-cadherin/beta-catenin complex were backed on track by RY10-4, and the expression of MMP-2/9 in MDA-MB-231, which functions as a major negative factor of ECM, was down-regulated after co-cultured with RY10-4. Furthermore the pathway related to MMP-2/9 and E-cadherin was assessed by the western blot. As the results showed, the MAPK pathway and the spread of beta-catenin were affected by RY10-4 to exert the anti-metastasis on MDA-MB-231. Collectively, the research revealed a novel anti-tumor ability of RY10-4 by inhibiting migration and invasion in MDA-MB-231.


