6-HydroxytropinoneCAS# 5932-53-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5932-53-6 | SDF | Download SDF |
| PubChem ID | 51346151 | Appearance | White to yellowish crystals |
| Formula | C8H13NO2 | M.Wt | 155.2 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in chloroform | ||
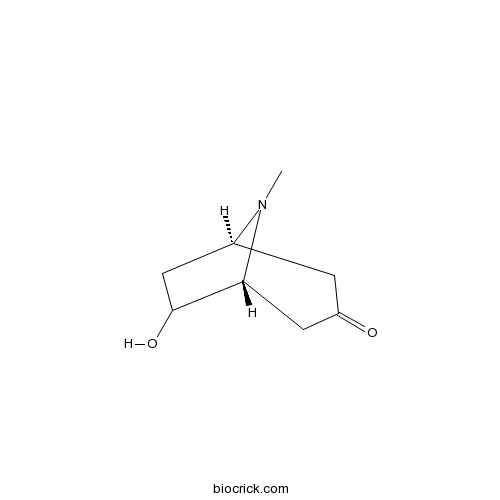
| Chemical Name | (1R,5R)-6-hydroxy-8-methyl-8-azabicyclo[3.2.1]octan-3-one | ||
| SMILES | CN1C2CC(C1CC(=O)C2)O | ||
| Standard InChIKey | UOHSTKWPZWFYTF-LSBSRIOGSA-N | ||
| Standard InChI | InChI=1S/C8H13NO2/c1-9-5-2-6(10)4-7(9)8(11)3-5/h5,7-8,11H,2-4H2,1H3/t5-,7+,8?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

6-Hydroxytropinone Dilution Calculator

6-Hydroxytropinone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4433 mL | 32.2165 mL | 64.433 mL | 128.866 mL | 161.0825 mL |
| 5 mM | 1.2887 mL | 6.4433 mL | 12.8866 mL | 25.7732 mL | 32.2165 mL |
| 10 mM | 0.6443 mL | 3.2216 mL | 6.4433 mL | 12.8866 mL | 16.1082 mL |
| 50 mM | 0.1289 mL | 0.6443 mL | 1.2887 mL | 2.5773 mL | 3.2216 mL |
| 100 mM | 0.0644 mL | 0.3222 mL | 0.6443 mL | 1.2887 mL | 1.6108 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Glucohesperin
Catalog No.:BCN8939
CAS No.:33049-17-1
- Sceleratine N-oxide
Catalog No.:BCN8938
CAS No.:103184-92-5
- Senecivernine N-oxide
Catalog No.:BCN8937
CAS No.:101687-28-9
- Riddelline N-oxide
Catalog No.:BCN8936
CAS No.:75056-11-0
- 7-O-Acetyllycopsamine N-oxide
Catalog No.:BCN8935
CAS No.:685132-58-5
- Merenskine N-oxide
Catalog No.:BCN8934
CAS No.:96657-95-3
- Merenskine
Catalog No.:BCN8933
CAS No.:96657-94-2
- Jacobine N-oxide
Catalog No.:BCN8932
CAS No.:38710-25-7
- Intermedine N-oxide
Catalog No.:BCN8931
CAS No.:95462-14-9
- Integerrimine N-oxide
Catalog No.:BCN8930
CAS No.:85955-28-8
- Heliotridine
Catalog No.:BCN8929
CAS No.:520-63-8
- Erucifolin N-oxide
Catalog No.:BCN8928
CAS No.:123864-94-8
- Glucoraphenin
Catalog No.:BCN8942
CAS No.:108844-81-1
- Heliosupine N-oxide
Catalog No.:BCN8943
CAS No.:31701-88-9
- Indicine hydrochloride
Catalog No.:BCN8945
CAS No.:1195140-94-3
- Merepoxine
Catalog No.:BCN8946
CAS No.:115777-94-1
- Homatropine
Catalog No.:BCN8948
CAS No.:87-00-3
- Echimidine N-oxide
Catalog No.:BCN8950
CAS No.:41093-89-4
- Lycopsamine N-oxide
Catalog No.:BCN8951
CAS No.:95462-15-0
- Usaramine N-oxide
Catalog No.:BCN8952
CAS No.:117020-54-9
- Scopolamine N-oxide hydrobromide
Catalog No.:BCN8953
CAS No.:6106-81-6
- Noratropine
Catalog No.:BCN8955
CAS No.:16839-98-8
- Glucoraphasatin
Catalog No.:BCN8957
CAS No.:28463-23-2
- Glucobrassicin
Catalog No.:BCN8958
CAS No.:143231-38-3
Enantiomeric high-performance liquid chromatography resolution and absolute configuration of 6beta-benzoyloxy-3alpha-tropanol.[Pubmed:27214755]
J Sep Sci. 2016 Jul;39(14):2720-7.
The absolute configuration of the naturally occurring isomers of 6beta-benzoyloxy-3alpha-tropanol (1) has been established by the combined use of chiral high-performance liquid chromatography with electronic circular dichroism detection and optical rotation detection. For this purpose (+/-)-1, prepared in two steps from racemic 6-Hydroxytropinone (4), was subjected to chiral high-performance liquid chromatography with electronic circular dichroism and optical rotation detection allowing the online measurement of both chiroptical properties for each enantiomer, which in turn were compared with the corresponding values obtained from density functional theory calculations. In an independent approach, preparative high-performance liquid chromatography separation using an automatic fraction collector, yielded an enantiopure sample of OR (+)-1 whose vibrational circular dichroism spectrum allowed its absolute configuration assignment when the bands in the 1100-950 cm(-1) region were compared with those of the enantiomers of esters derived from 3alpha,6beta-tropanediol. In addition, an enantiomerically enriched sample of 4, instead of OR (+/-)-4, was used for the same transformation sequence, whose high-performance liquid chromatography follow-up allowed their spectroscopic correlation. All evidences lead to the OR (+)-(1S,3R,5S,6R) and OR (-)-(1R,3S,5R,6S) absolute configurations, from where it follows that samples of 1 isolated from Knightia strobilina and Erythroxylum zambesiacum have the OR (+)-(1S,3R,5S,6R) absolute configuration, while the sample obtained from E. rotundifolium has the OR (-)-(1R,3S,5R,6S) absolute configuration.


