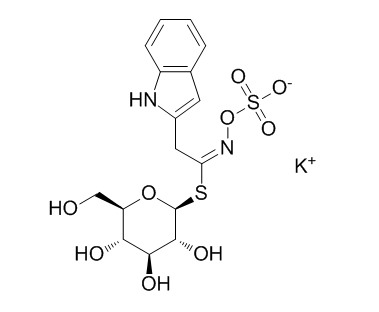
GlucobrassicinCAS# 143231-38-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 143231-38-3 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | White-pink powder |
| Formula | C16H19KN2O9S2 | M.Wt | 486.6 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | 3-Indolylmethylglucosinolate potassium salt | ||
| Solubility | Soluble in methan | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Glucobrassicin Dilution Calculator

Glucobrassicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0551 mL | 10.2754 mL | 20.5508 mL | 41.1015 mL | 51.3769 mL |
| 5 mM | 0.411 mL | 2.0551 mL | 4.1102 mL | 8.2203 mL | 10.2754 mL |
| 10 mM | 0.2055 mL | 1.0275 mL | 2.0551 mL | 4.1102 mL | 5.1377 mL |
| 50 mM | 0.0411 mL | 0.2055 mL | 0.411 mL | 0.822 mL | 1.0275 mL |
| 100 mM | 0.0206 mL | 0.1028 mL | 0.2055 mL | 0.411 mL | 0.5138 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Glucoraphasatin
Catalog No.:BCN8957
CAS No.:28463-23-2
- Noratropine
Catalog No.:BCN8955
CAS No.:16839-98-8
- Scopolamine N-oxide hydrobromide
Catalog No.:BCN8953
CAS No.:6106-81-6
- Usaramine N-oxide
Catalog No.:BCN8952
CAS No.:117020-54-9
- Lycopsamine N-oxide
Catalog No.:BCN8951
CAS No.:95462-15-0
- Echimidine N-oxide
Catalog No.:BCN8950
CAS No.:41093-89-4
- Homatropine
Catalog No.:BCN8948
CAS No.:87-00-3
- Merepoxine
Catalog No.:BCN8946
CAS No.:115777-94-1
- Indicine hydrochloride
Catalog No.:BCN8945
CAS No.:1195140-94-3
- Heliosupine N-oxide
Catalog No.:BCN8943
CAS No.:31701-88-9
- Glucoraphenin
Catalog No.:BCN8942
CAS No.:108844-81-1
- 6-Hydroxytropinone
Catalog No.:BCN8940
CAS No.:5932-53-6
- Glucocheirolin
Catalog No.:BCN8959
CAS No.:15592-36-6
- Sinalbin potassium salt
Catalog No.:BCN8960
CAS No.:16411-05-5
- Gluconasturtiin
Catalog No.:BCN8961
CAS No.:18425-76-8
- Glucohirsutin
Catalog No.:BCN8962
CAS No.:21973-60-4
- Glucoraphasatin potassium salt
Catalog No.:BCN8963
CAS No.:245550-64-5
- Glucocamelinin
Catalog No.:BCN8964
CAS No.:67884-10-0
- Glucoarabin
Catalog No.:BCN8965
CAS No.:67920-64-3
- 4-Methoxyglucobrassicin
Catalog No.:BCN8966
CAS No.:83327-21-3
- 4-Hydroxyglucobrassicin
Catalog No.:BCN8967
CAS No.:83327-20-2
- Epiprogoitrin
Catalog No.:BCN8968
CAS No.:21087-74-1
- Phyllalbine
Catalog No.:BCN8969
CAS No.:4540-25-4
- Glucobarbarin
Catalog No.:BCN8970
CAS No.:21087-78-5
Dietary AhR Ligands Regulate AhRR Expression in Intestinal Immune Cells and Intestinal Microbiota Composition.[Pubmed:32366032]
Int J Mol Sci. 2020 Apr 30;21(9). pii: ijms21093189.
A diet rich in vegetables and fruit is generally considered healthy because of a high content of phytochemicals, vitamins, and fiber. The phytochemical indole-3-carbinol (I3C), a derivative of Glucobrassicin, is sold as a dietary supplement promising diverse health benefits. I3C metabolites act as ligands of the aryl hydrocarbon receptor (AhR), an important sensor for environmental polyaromatic chemicals. Here, we investigated how dietary AhR ligand supplementation influences AhR target gene expression and intestinal microbiota composition. For this, we used AhR repressor (AhRR)-reporter mice as a tool to study AhR activation in the intestine following dietary I3C-supplementation in comparison with AhR ligand-deprived diets, including a high fat diet. AhRR expression in intestinal immune cells was mainly driven by dietary AhR ligands and was independent of microbial metabolites. A lack of dietary AhR ligands caused enhanced susceptibility to dextran sodium sulfate (DSS)-induced colitis and correlated with the expansion of Enterobacteriaceae, whereas Clostridiales, Muribaculaceae, and Rikenellaceae were strongly reduced. I3C supplementation largely reverted this effect. Comparison of I3C-induced changes in microbiota composition using wild-type (WT), AhRR-deficient, and AhR-deficient mice revealed both AhR-dependent and -independent alterations in the microbiome. Overall, our study demonstrates that dietary AhR ligand supplementation has a profound influence on Ahrr expression in intestinal immune cells as well as microbiota composition.
Profiling of Individual Desulfo-Glucosinolate Content in Cabbage Head (Brassica oleracea var. capitata) Germplasm.[Pubmed:32316621]
Molecules. 2020 Apr 17;25(8). pii: molecules25081860.
Individual glucosinolates (GSLs) were assessed to select cabbage genotypes for a potential breeding program. One hundred forty-six cabbage genotypes from different origins were grown in an open field from March to June 2019; the cabbage heads were used for GSL analyses. Seven aliphatics [glucoiberin (GIB), progoitrin (PRO), epi-progoitrin (EPI), sinigrin (SIN), glucoraphanin (GRA), glucoerucin (GER) and gluconapin (GNA)], one aromatic [gluconasturtiin (GNS)] and four indolyl GSLs [Glucobrassicin (GBS), 4-hydroxyGlucobrassicin (4HGBS), 4-methoxyGlucobrassicin (4MGBS), neoGlucobrassicin (NGBS)] were found this study. Significant variation was observed in the individual GSL content and in each class of GSLs among the cabbage genotypes. Aliphatic GSLs were predominant (58.5%) among the total GSLs, followed by indolyl GSL (40.7%) and aromatic GSLs (0.8%), showing 46.4, 51.2 and 137.8% coefficients of variation, respectively. GIB, GBS and NGBS were the most common GSLs found in all genotypes. GBS was the most dominant GSL, with an average value of 3.91 micromol g(-1) (0.79 to 13.14 micromol g(-1)). SIN, GIB, PRO and GRA were the other major GSLs, showing average values of 3.45, 1.50, 0.77 and 0.62 micromol g(-1), respectively. The genotypes with relatively high contents of GBS, SIN, GIB and GRA warrant detailed studies for future breeding programs since the hydrolysis products of these GSLs have several anti-cancer properties.
Comparative study of the phytochemical and mineral composition of fresh and cooked broccolini.[Pubmed:32036908]
Food Res Int. 2020 Mar;129:108798.
Broccolini is originated from crossing the regular broccoli with Chinese kale. Consequently, it has similar properties to these vegetables, but other very particular characteristics. Its consumption has increased in the last few years and, consequently, there have been some studies related to its quality parameters and the influence of different cooking methods. Nevertheless, changes on its phenolic composition and mineral content originated by its cooking have not been investigated in-depth so far. Here we report the phytochemical profile of broccolini before and after boiling, steaming, and griddling cooking treatments. The mineral content and phytochemicals were assessed by inductively coupled plasma-mass spectrometry (ICP-MS) and high performance liquid chromatography-mass spectrometry (HPLC-MS), respectively. The main phenolics (mainly hydroxycinnamic acid derivatives from caffeic, coumaric, ferulic and sinapic acids) were quantified. Three oxylipins, three flavonoid glycosides and the glucosinolate Glucobrassicin were also identified. ABTS and DPPH assays were also used as screening methods to assess the antioxidant potential of broccolini. A significant loss of the phenolic compounds and a reduction of the antioxidant activity were observed after the three cooking methods. Clear disadvantages were detected when broccolini was boiled, namely high losses of phenolic acids and derivatives (70%). Steaming and griddling also led to a significant loss of phenolics (50%) from fresh broccolini. The mineral content of this vegetable after domestic cooking procedures is also reported for the first time, calculating the contribution of broccolini consumption to official daily recommendations.
Evolution of important glucosinolates in three common Brassica vegetables during their processing into vegetable powder and in vitro gastric digestion.[Pubmed:31915766]
Food Funct. 2020 Jan 29;11(1):211-220.
Evolution of important glucosinolates (GLSs), namely, sinigrin, glucoraphanin, glucoerucin and Glucobrassicin, in three commonly consumed Brassica vegetables viz. white cabbage, Chinese cabbage and bok choy during their processing into vegetable powder was investigated. Drying was noted to be a major processing step causing significant losses of GLSs. Interestingly, different GLSs and even the same GLSs in different vegetables showed different thermal stabilities during drying. The stability of GLSs in vegetable powder during in vitro gastric digestion was also studied. Glucoraphanin exhibited the highest stability while Glucobrassicin was the most vulnerable GLS under in vitro gastric conditions. White cabbage is found to be a promising material for the production of vegetable powder as it contains high contents of GLSs, especially glucoraphanin and glucoerucin, which are important precursors of anticarcinogenic compounds, namely sulforaphane and erucin. These two GLSs were also noted to be stable during in vitro gastric digestion.
Health-promoting phytochemicals and antioxidant capacity in different organs from six varieties of Chinese kale.[Pubmed:31889076]
Sci Rep. 2019 Dec 30;9(1):20344.
Chinese kale (Brassica oleracea var. alboglabra) has high nutritional value. This study investigated the contents of glucosinolates, antioxidants (chlorophylls, carotenoids, vitamin C, and total phenolics), and antioxidant capacity in five organs from six varieties of Chinese kale. The highest concentrations of individual and total glucosinolates were in the roots and inflorescences, respectively. The highest levels of antioxidants and antioxidant capacity were in inflorescences and leaves. Plant organs played a predominant role in glucosinolate and antioxidant accumulation. Glucoiberin, glucoraphanin, and Glucobrassicin, the main anticarcinogenic glucosinolates, could be enhanced simultaneously because of their high positive correlations. The relationship between glucosinolates and antioxidant capacity indicated that Glucobrassicin might contribute to the total antioxidant capacity. These results provide useful information related to consumption, breeding of functional varieties, and use of the non-edible organs of Chinese kale.
Use of elicitation in the cultivation of Bimi(R) for food and ingredients.[Pubmed:31875967]
J Sci Food Agric. 2020 Mar 30;100(5):2099-2109.
BACKGROUND: Cruciferous foods rich in health-promoting metabolites are of particular interest to consumers as well as being a good source of bioactives-enriched ingredients. Several elicitors have been used to stimulate the biosynthesis and accumulation of secondary metabolites in foods; however, little is known about the response of new hybrid varieties, such as Bimi(R), under field-crop production conditions. Therefore, this study was designed to evaluate the effect of salicylic acid (200 mumol L(-1) , SA), methyl jasmonate (100 mumol L(-1) , MeJA), and their combination on Bimi plant organs (inflorescences and aerial vegetative tissues - stems and leaves). For this, the composition of the glucosinolates present in the tissues was evaluated. Also, aqueous extracts of the plant material, obtained with different times of extraction with boiling water, were studied. RESULTS: The results indicate that the combined treatment (SA + MeJA) significantly increased the content of glucosinolates in the inflorescences and that MeJA was the most effective elicitor in leaves. Regarding the aqueous extracts, the greatest amount of glucosinolates was extracted at 30 min - except for the leaves elicited with MeJA, for which 15 min was optimal. CONCLUSION: The elicitation in the field enriched leaves in Glucobrassicin (GB), 4-methoxyGlucobrassicin (MGB), and neoGlucobrassicin (NGB) and stems and inflorescences in glucoraphanin, 4-hydroxyGlucobrassicin, GB, MGB, and NGB. In this way, this enhanced vegetable material favored the presence of bioactives in the extracts, which is of great interest regarding enriched foods and ingredients with added value obtained from them. (c) 2019 Society of Chemical Industry.
Hydroxyl Radical Scavenging of Indole-3-Carbinol: A Mechanistic and Kinetic Study.[Pubmed:31763562]
ACS Omega. 2019 Nov 8;4(21):19375-19381.
Indole-3-carbinol (I3C) is the product of the enzymatic hydrolysis of Glucobrassicin in the human body. I3C exhibits diverse bioactivities. It is used as a supplement to enhance the efficiency of some cancer therapies and is available as an over-the-counter dietary supplement described as a potential antioxidant, among other health benefits. Thus, it is important to develop an in-depth understanding of its antioxidant activity. In this study, the hydroxyl radical scavenging of I3C has been investigated in silico under physiologically relevant conditions (aqueous and lipid-mimetic pentyl ethanoate environment) using thermochemical and kinetic calculations. For benchmarking purposes, the results were compared to known experimental data. The overall reaction rate constant of the HO(*) radical scavenging of I3C in water was found to be 2.30 x 10(10) M(-1) s(-1) and over two times lower in lipid-mimetic pentyl ethanoate solvent at 7.74 x 10(9) M(-1) s(-1). The results also highlighted that the HO(*) radical scavenging follows almost exclusively the radical adduct formation mechanism (>94%) in a lipid mimetic medium, whereas this mechanism contributes about 60% in aqueous environments. I3C is considered a dopamine-like antioxidant, its main function being prevention of oxidative degradation of lipids; our study supports this view.
Effect of pretreatment on bioactive compounds in wild rocket juice.[Pubmed:31749470]
J Food Sci Technol. 2019 Dec;56(12):5234-5242.
The aim of the study was to determine the effect of pretreatment with hot water or steaming on glucosinolates, polyphenols contents and antioxidant capacity in obtained raw juices. Moreover, in vitro cytotoxic activity of the raw juice to the cells derived from the gastrointestinal tract, including the small intestine (IEC-6 cell line), colon (Caco-2 cell line) and the liver (HepG2 cell line) were also investigated. The dominant glucosinolates in the wild rocket leaves were glucoraphanin (36%) and dimeric 4-mercaptobutyl (30%), followed by glucosativin and glucoerucin, 11% per each. Glucothiobeinin (6%), Glucobrassicin (1%), 4-methoxyGlucobrassicin (1%) and two unidentified compounds (4%) were also detected in rocket leaves. In terms of phenolic compounds, quercetin constituted the majority (55%) and the rest composed of hydroxycinnamic acids. In raw juices produced from steamed, pretreatment with hot water and untreated (control) leaves, glucosinolate contents were lower about 21%, 37% and 53%, respectively, than their levels in the raw material. The highest content of polyphenols among the juices tested (45.4 mg/100 g fresh weight) and antioxidant capacity (5.8 micromol Trolox/1 g f.w.) was recorded in the raw juice from pretreated leaves with hot water. The wild rocket raw juice concentrations responsible for a 50% reduction in Caco-2 and HepG2 cell viability were estimated at 1.87 +/- 0.08 mg/mL and 3.54 +/- 0.29 mg/mL. The viability of the IEC-6 cells was reduced by only 19.04%, at the maximum concentration (3.6 mg/mL) of the raw juice.
Glucobrassicin Metabolites Ameliorate the Development of Portal Hypertension and Cirrhosis in Bile Duct-Ligated Rats.[Pubmed:31454890]
Int J Mol Sci. 2019 Aug 26;20(17). pii: ijms20174161.
Patients suffering from liver cirrhosis are often complicated with the formation of portosystemic collateral vessels, which is associated with the progression of a splanchnic hyperdynamic circulatory state. Alleviating pathological angiogenesis has thus been proposed to be a feasible treatment strategy. Indole-3-carbinol (C9H9NO, I3C) and 3,3'-diindolymethane (DIM), formed by the breakdown of glucosinolate Glucobrassicin, are prevalent in cruciferous vegetables and have anti-angiogenesis properties. We aimed to evaluate their influences on portal hypertension, the severity of mesenteric angiogenesis, and portosystemic collaterals in cirrhosis. Sprague-Dawley rats with common bile duct ligation (CBDL)-induced liver cirrhosis or sham operation (surgical control) were randomly allocated to receive I3C (20 mg/kg/3 day), DIM (5 mg/kg/day) or vehicle for 28 days. The systemic and portal hemodynamics, severity of portosystemic shunting, mesenteric angiogenesis, and mesenteric proangiogenic factors protein expressions were evaluated. Compared to vehicle, both DIM and I3C significantly reduced portal pressure, ameliorated liver fibrosis, and down-regulated mesenteric protein expressions of vascular endothelial growth factor and phosphorylated Akt. DIM significantly down-regulated pErk, and I3C down-regulated NFkappaB, pIkappaBalpha protein expressions, and reduced portosystemic shunting degree. The cruciferous vegetable byproducts I3C and DIM not only exerted a portal hypotensive effect but also ameliorated abnormal angiogenesis and portosystemic collaterals in cirrhotic rats.
Variability of Bioactive Glucosinolates, Isothiocyanates and Enzyme Patterns in Horseradish Hairy Root Cultures Initiated from Different Organs.[Pubmed:31382520]
Molecules. 2019 Aug 2;24(15). pii: molecules24152828.
Horseradish hairy root cultures are suitable plant tissue organs to study the glucosinolate-myrosinase-isothiocyanate system and also to produce the biologically active isothiocyanates and horseradish peroxidase, widely used in molecular biology. Fifty hairy root clones were isolated after Agrobacterium rhizogenes infection of surface sterilized Armoracia rusticana petioles and leaf blades, from which 21 were viable after antibiotic treatment. Biomass properties (e.g. dry weight %, daily growth index), glucosinolate content (analyzed by liquid chromatography-electronspray ionization-mass spectrometry (LC-ESI-MS/MS)), isothiocyanate and nitrile content (analyzed by gas chromatography-mass spectrometry (GC-MS)), myrosinase (on-gel detection) and horseradish peroxidase enzyme patterns (on-gel detection and spectrophotometry), and morphological features were examined with multi-variable statistical analysis. In addition to the several positive and negative correlations, the most outstanding phenomenon was many parameters of the hairy root clones showed dependence on the organ of origin. Among others, the daily growth index, sinigrin, Glucobrassicin, 3-phenylpropionitrile, indole-3-acetonitrile and horseradish peroxidase values showed significantly higher levels in horseradish hairy root cultures initiated from leaf blades.
Effect of Cooking Method on Antioxidant Compound Contents in Cauliflower.[Pubmed:31328127]
Prev Nutr Food Sci. 2019 Jun;24(2):210-216.
In this study, we determined the contents of glucosinolate, polyphenol, and flavonoid, and the antioxidant activities of uncooked, steamed, and boiled cauliflower. Eight glucosinolate peaks were detected, representing glucoiberin, progoitrin, glucoraphanin, sinigrin, gluconapin, glucoiberverin, Glucobrassicin, and gluconasturtiin. Boiled cauliflower contained significantly lowered concentrations of glucosinolate, total polyphenol, and total flavonoid compared to uncooked or steamed cauliflower. These results clearly indicate that health-promoting compounds in cauliflower are significantly impacted by different cooking methods: uncooked> steamed> boiled. The amounts of total polyphenols and total flavonoids in uncooked cauliflower extracted with 80% ethanol were higher than extracts of steamed and boiled cauliflower. The highest antioxidant activity was observed in uncooked cauliflower extracted using 80% ethanol compared to those extracted with water at the same concentration. Steamed and boiled cauliflower extracts also showed lower antioxidant activity than uncooked extracts. Based on these results, fresh uncooked cauliflower contains higher contents of health-promoting compounds and elevated antioxidant activity. Moreover, steaming may be more desirable than boiling in order to minimize loss of glucosinolates when storing, pretreating, processing, and cooking cruciferous vegetables.
Glucosinolate Content and Sensory Evaluation of Baby Leaf Rapeseed from Annual and Biennial White- and Yellow-Flowering Cultivars with Repeated Harvesting in Two Seasons.[Pubmed:31237979]
J Food Sci. 2019 Jul;84(7):1888-1899.
The chemical and sensory quality of field-grown vegetables may be influenced by cultivar choice and agronomic factors but knowledge is lacking on the new rapeseed vegetables. White- and yellow-flowering rapeseed cultivars were tested in two seasonally different field studies in Denmark at three different growing stages by early sowing the first year and late sowing the second year. Content of glucosinolates (GLSs) was analyzed, and the sensory quality of baby leaf samples was evaluated. The GLS content differed among cultivars across years in all growing stages, with biennial cultivars having the highest GLS content. In the second year, a higher content of all identified GLSs was found at two growing stages except for neoGlucobrassicin and gluconasturtiin, compared to the first year. On the contrary, higher contents of all identified GLSs were found at a third stage in the first year except for progoitrin and 4-methoxy Glucobrassicin. Sensory evaluation of bitterness revealed differences among cultivars, higher intensities of bitterness in biennial cultivars, and a relationship between bitterness and content of bitter-tasting and total GLSs. The effect of repeated harvesting on GLS content differed between the years and no general pattern was seen, except that the composition of individual GLSs was comparable for the biennial cultivars. We conclude that growing season and life cycle had a stronger influence on GLS content than stage at harvest. The link between bitter-tasting GLSs and bitterness revealed that life cycle and seasonal effects affected the sensory profile of baby leaf rapeseed thereby making a healthier product due to high content of health-beneficial GLSs.
Comparative analysis of glucosinolate production in hairy roots of green and red kale (Brassica oleracea var. acephala).[Pubmed:31124740]
Prep Biochem Biotechnol. 2019;49(8):775-782.
Glucosinolates (GSLs) are sulfur- and nitrogen-containing secondary metabolites that function in plant defense and provide benefits to human health. In this study, using Agrobacterium rhizogenes R1000, green and red kale hairy roots were established. The expression levels of GSLs biosynthesis genes and their accumulation in both kale hairy roots were analyzed by quantitative real-time PCR and HPLC. The results showed that the expression of most indolic GSLs biosynthesis genes was higher in the hairy roots of green kale than in that of red kale. In contrast, the expression of BoCYP83A1 and BoSUR1 encoding key enzymes aromatic GSL biosynthesis was significantly higher in red kale hairy root. The HPLC analysis identified six GSLs. The levels of 4-methoxyGlucobrassicin, Glucobrassicin, and 4-hydroxyGlucobrassicin were 6.21, 5.98, and 2 times higher, respectively, in green kale than in red kale, whereas the levels of neoGlucobrassicin and gluconasturtiin were 16.2 and 3.48 times higher, respectively, in red kale than in green kale. Our study provides insights into the underlying mechanisms of GSLs biosynthesis in kale hairy roots and can be potentially used as "biological factories" for producing bioactive substances such as GSLs.


