GluconasturtiinCAS# 18425-76-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18425-76-8 | SDF | Download SDF |
| PubChem ID | 24721260 | Appearance | White to beige powder |
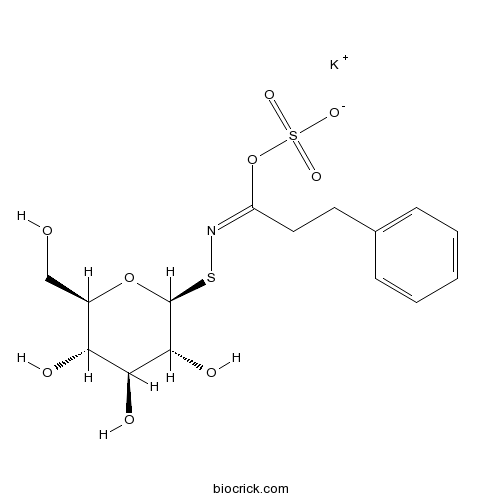
| Formula | C15H20KNO9S2 | M.Wt | 461.6 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | 2-Phenylethylglucosinolate potassium salt | ||
| Solubility | Soluble in methanol and water | ||
| Chemical Name | potassium;[(E)-C-(2-phenylethyl)-N-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanylcarbonimidoyl] sulfate | ||
| SMILES | C1=CC=C(C=C1)CCC(=NSC2C(C(C(C(O2)CO)O)O)O)OS(=O)(=O)[O-].[K+] | ||
| Standard InChIKey | KNQJXSDRCFVICE-SWKZEUNZSA-M | ||
| Standard InChI | InChI=1S/C15H21NO9S2.K/c17-8-10-12(18)13(19)14(20)15(24-10)26-16-11(25-27(21,22)23)7-6-9-4-2-1-3-5-9;/h1-5,10,12-15,17-20H,6-8H2,(H,21,22,23);/q;+1/p-1/b16-11+;/t10-,12-,13+,14-,15+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Gluconasturtiin Dilution Calculator

Gluconasturtiin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1664 mL | 10.8319 mL | 21.6638 mL | 43.3276 mL | 54.1594 mL |
| 5 mM | 0.4333 mL | 2.1664 mL | 4.3328 mL | 8.6655 mL | 10.8319 mL |
| 10 mM | 0.2166 mL | 1.0832 mL | 2.1664 mL | 4.3328 mL | 5.4159 mL |
| 50 mM | 0.0433 mL | 0.2166 mL | 0.4333 mL | 0.8666 mL | 1.0832 mL |
| 100 mM | 0.0217 mL | 0.1083 mL | 0.2166 mL | 0.4333 mL | 0.5416 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sinalbin potassium salt
Catalog No.:BCN8960
CAS No.:16411-05-5
- Glucocheirolin
Catalog No.:BCN8959
CAS No.:15592-36-6
- Glucobrassicin
Catalog No.:BCN8958
CAS No.:143231-38-3
- Glucoraphasatin
Catalog No.:BCN8957
CAS No.:28463-23-2
- Noratropine
Catalog No.:BCN8955
CAS No.:16839-98-8
- Scopolamine N-oxide hydrobromide
Catalog No.:BCN8953
CAS No.:6106-81-6
- Usaramine N-oxide
Catalog No.:BCN8952
CAS No.:117020-54-9
- Lycopsamine N-oxide
Catalog No.:BCN8951
CAS No.:95462-15-0
- Echimidine N-oxide
Catalog No.:BCN8950
CAS No.:41093-89-4
- Homatropine
Catalog No.:BCN8948
CAS No.:87-00-3
- Merepoxine
Catalog No.:BCN8946
CAS No.:115777-94-1
- Indicine hydrochloride
Catalog No.:BCN8945
CAS No.:1195140-94-3
- Glucohirsutin
Catalog No.:BCN8962
CAS No.:21973-60-4
- Glucoraphasatin potassium salt
Catalog No.:BCN8963
CAS No.:245550-64-5
- Glucocamelinin
Catalog No.:BCN8964
CAS No.:67884-10-0
- Glucoarabin
Catalog No.:BCN8965
CAS No.:67920-64-3
- 4-Methoxyglucobrassicin
Catalog No.:BCN8966
CAS No.:83327-21-3
- 4-Hydroxyglucobrassicin
Catalog No.:BCN8967
CAS No.:83327-20-2
- Epiprogoitrin
Catalog No.:BCN8968
CAS No.:21087-74-1
- Phyllalbine
Catalog No.:BCN8969
CAS No.:4540-25-4
- Glucobarbarin
Catalog No.:BCN8970
CAS No.:21087-78-5
- Glucolimnanthin
Catalog No.:BCN8971
CAS No.:111810-95-8
- Glucocapparin
Catalog No.:BCN8972
CAS No.:15592-33-3
- Sinalbin
Catalog No.:BCN8973
CAS No.:20196-67-2
Profiling of Individual Desulfo-Glucosinolate Content in Cabbage Head (Brassica oleracea var. capitata) Germplasm.[Pubmed:32316621]
Molecules. 2020 Apr 17;25(8). pii: molecules25081860.
Individual glucosinolates (GSLs) were assessed to select cabbage genotypes for a potential breeding program. One hundred forty-six cabbage genotypes from different origins were grown in an open field from March to June 2019; the cabbage heads were used for GSL analyses. Seven aliphatics [glucoiberin (GIB), progoitrin (PRO), epi-progoitrin (EPI), sinigrin (SIN), glucoraphanin (GRA), glucoerucin (GER) and gluconapin (GNA)], one aromatic [Gluconasturtiin (GNS)] and four indolyl GSLs [glucobrassicin (GBS), 4-hydroxyglucobrassicin (4HGBS), 4-methoxyglucobrassicin (4MGBS), neoglucobrassicin (NGBS)] were found this study. Significant variation was observed in the individual GSL content and in each class of GSLs among the cabbage genotypes. Aliphatic GSLs were predominant (58.5%) among the total GSLs, followed by indolyl GSL (40.7%) and aromatic GSLs (0.8%), showing 46.4, 51.2 and 137.8% coefficients of variation, respectively. GIB, GBS and NGBS were the most common GSLs found in all genotypes. GBS was the most dominant GSL, with an average value of 3.91 micromol g(-1) (0.79 to 13.14 micromol g(-1)). SIN, GIB, PRO and GRA were the other major GSLs, showing average values of 3.45, 1.50, 0.77 and 0.62 micromol g(-1), respectively. The genotypes with relatively high contents of GBS, SIN, GIB and GRA warrant detailed studies for future breeding programs since the hydrolysis products of these GSLs have several anti-cancer properties.
Induction of Apoptosis by Gluconasturtiin-Isothiocyanate (GNST-ITC) in Human Hepatocarcinoma HepG2 Cells and Human Breast Adenocarcinoma MCF-7 Cells.[Pubmed:32182965]
Molecules. 2020 Mar 9;25(5). pii: molecules25051240.
Gluconasturtiin, a glucosinolate present in watercress, is hydrolysed by myrosinase to form Gluconasturtiin-isothiocyanate (GNST-ITC), which has potential chemopreventive effects; however, the underlying mechanisms of action have not been explored, mainly in human cell lines. The purpose of the study is to evaluate the cytotoxicity of GNST-ITC and to further assess its potential to induce apoptosis. GNST-ITC inhibited cell proliferation in both human hepatocarcinoma (HepG2) and human breast adenocarcinoma (MCF-7) cells with IC50 values of 7.83 microM and 5.02 microM, respectively. Morphological changes as a result of GNST-ITC-induced apoptosis showed chromatin condensation, nuclear fragmentation, and membrane blebbing. Additionally, Annexin V assay showed proportion of cells in early and late apoptosis upon exposure to GNST-ITC in a time-dependent manner. To delineate the mechanism of apoptosis, cell cycle arrest and expression of caspases were studied. GNST-ITC induced a time-dependent G2/M phase arrest, with reduction of 82% and 93% in HepG2 and MCF-7 cell lines, respectively. The same treatment also led to the subsequent expression of caspase-3/7 and -9 in both cells demonstrating mitochondrial-associated cell death. Collectively, these results reveal that GNST-ITC can inhibit cell proliferation and can induce cell death in HepG2 and MCF-7 cancer cells via apoptosis, highlighting its potential development as an anticancer agent.
Assessing the Fate and Bioavailability of Glucosinolates in Kale (Brassica oleracea) Using Simulated Human Digestion and Caco-2 Cell Uptake Models.[Pubmed:31374175]
J Agric Food Chem. 2019 Aug 28;67(34):9492-9500.
Glucosinolates and their hydrolysis products were characterized in fresh and in in vitro gastric and intestinal digesta of Dinosaur kale (Brassica oleracea L var. palmifolia DC). In fresh kale, glucoraphanin, sinigrin, gluconapin, Gluconasturtiin, glucoerucin, glucobrasscin, and 4-methoxylglucobrassicin were identified. After 120 min of gastric digestion, the levels of glucoraphanin, sinigrin, and gluconapin decreased, and no glucoerucin or glucobrasscin was detected. However, a concomitant increase in the glucosinolate hydrolysis products allyl nitrile, 3-butenyl isothiocyanate, phenylacetonitrile, and sulforaphane was observed. This trend continued through intestinal digestion. After 120 min, the levels of allyl nitrile, 3-butenyl isothiocyanate, phenylacetonitrile, and sulforaphane were 88.19 +/- 5.85, 222.15 +/- 30.26, 129.17 +/- 17.57, and 13.71 +/- 0.62 pmol/g fresh weight, respectively. Intestinal digesta were then applied to Caco-2 cell monolayers to assess the bioavailability. After 6 h of incubation, no glucosinolates were detected and the percentage of total cellular uptake of the glucosinolate hydrolysis products ranged from 29.35% (sulforaphane) to 46.60% (allyl nitrile).
Effect of Cooking Method on Antioxidant Compound Contents in Cauliflower.[Pubmed:31328127]
Prev Nutr Food Sci. 2019 Jun;24(2):210-216.
In this study, we determined the contents of glucosinolate, polyphenol, and flavonoid, and the antioxidant activities of uncooked, steamed, and boiled cauliflower. Eight glucosinolate peaks were detected, representing glucoiberin, progoitrin, glucoraphanin, sinigrin, gluconapin, glucoiberverin, glucobrassicin, and Gluconasturtiin. Boiled cauliflower contained significantly lowered concentrations of glucosinolate, total polyphenol, and total flavonoid compared to uncooked or steamed cauliflower. These results clearly indicate that health-promoting compounds in cauliflower are significantly impacted by different cooking methods: uncooked> steamed> boiled. The amounts of total polyphenols and total flavonoids in uncooked cauliflower extracted with 80% ethanol were higher than extracts of steamed and boiled cauliflower. The highest antioxidant activity was observed in uncooked cauliflower extracted using 80% ethanol compared to those extracted with water at the same concentration. Steamed and boiled cauliflower extracts also showed lower antioxidant activity than uncooked extracts. Based on these results, fresh uncooked cauliflower contains higher contents of health-promoting compounds and elevated antioxidant activity. Moreover, steaming may be more desirable than boiling in order to minimize loss of glucosinolates when storing, pretreating, processing, and cooking cruciferous vegetables.
Glucosinolate Content and Sensory Evaluation of Baby Leaf Rapeseed from Annual and Biennial White- and Yellow-Flowering Cultivars with Repeated Harvesting in Two Seasons.[Pubmed:31237979]
J Food Sci. 2019 Jul;84(7):1888-1899.
The chemical and sensory quality of field-grown vegetables may be influenced by cultivar choice and agronomic factors but knowledge is lacking on the new rapeseed vegetables. White- and yellow-flowering rapeseed cultivars were tested in two seasonally different field studies in Denmark at three different growing stages by early sowing the first year and late sowing the second year. Content of glucosinolates (GLSs) was analyzed, and the sensory quality of baby leaf samples was evaluated. The GLS content differed among cultivars across years in all growing stages, with biennial cultivars having the highest GLS content. In the second year, a higher content of all identified GLSs was found at two growing stages except for neoglucobrassicin and Gluconasturtiin, compared to the first year. On the contrary, higher contents of all identified GLSs were found at a third stage in the first year except for progoitrin and 4-methoxy glucobrassicin. Sensory evaluation of bitterness revealed differences among cultivars, higher intensities of bitterness in biennial cultivars, and a relationship between bitterness and content of bitter-tasting and total GLSs. The effect of repeated harvesting on GLS content differed between the years and no general pattern was seen, except that the composition of individual GLSs was comparable for the biennial cultivars. We conclude that growing season and life cycle had a stronger influence on GLS content than stage at harvest. The link between bitter-tasting GLSs and bitterness revealed that life cycle and seasonal effects affected the sensory profile of baby leaf rapeseed thereby making a healthier product due to high content of health-beneficial GLSs.
Resolution of quantitative resistance to clubroot into QTL-specific metabolic modules.[Pubmed:31145785]
J Exp Bot. 2019 Oct 15;70(19):5375-5390.
Plant disease resistance is often under quantitative genetic control. Thus, in a given interaction, plant cellular responses to infection are influenced by resistance or susceptibility alleles at different loci. In this study, a genetic linkage analysis was used to address the complexity of the metabolic responses of Brassica napus roots to infection by Plasmodiophora brassicae. Metabolome profiling and pathogen quantification in a segregating progeny allowed a comparative mapping of quantitative trait loci (QTLs) involved in resistance and in metabolic adjustments. Distinct metabolic modules were associated with each resistance QTL, suggesting the involvement of different underlying cellular mechanisms. This approach highlighted the possible role of Gluconasturtiin and two unknown metabolites in the resistance conferred by two QTLs on chromosomes C03 and C09, respectively. Only two susceptibility biomarkers (glycine and glutathione) were simultaneously linked to the three main resistance QTLs, suggesting the central role of these compounds in the interaction. By contrast, several genotype-specific metabolic responses to infection were genetically unconnected to resistance or susceptibility. Likewise, variations of root sugar profiles, which might have influenced pathogen nutrition, were not found to be related to resistance QTLs. This work illustrates how genetic metabolomics can help to understand plant stress responses and their possible links with disease.
Comparative analysis of glucosinolate production in hairy roots of green and red kale (Brassica oleracea var. acephala).[Pubmed:31124740]
Prep Biochem Biotechnol. 2019;49(8):775-782.
Glucosinolates (GSLs) are sulfur- and nitrogen-containing secondary metabolites that function in plant defense and provide benefits to human health. In this study, using Agrobacterium rhizogenes R1000, green and red kale hairy roots were established. The expression levels of GSLs biosynthesis genes and their accumulation in both kale hairy roots were analyzed by quantitative real-time PCR and HPLC. The results showed that the expression of most indolic GSLs biosynthesis genes was higher in the hairy roots of green kale than in that of red kale. In contrast, the expression of BoCYP83A1 and BoSUR1 encoding key enzymes aromatic GSL biosynthesis was significantly higher in red kale hairy root. The HPLC analysis identified six GSLs. The levels of 4-methoxyglucobrassicin, glucobrassicin, and 4-hydroxyglucobrassicin were 6.21, 5.98, and 2 times higher, respectively, in green kale than in red kale, whereas the levels of neoglucobrassicin and Gluconasturtiin were 16.2 and 3.48 times higher, respectively, in red kale than in green kale. Our study provides insights into the underlying mechanisms of GSLs biosynthesis in kale hairy roots and can be potentially used as "biological factories" for producing bioactive substances such as GSLs.
Combined effect of ultrasound treatment and exogenous phytohormones on the accumulation of bioactive compounds in broccoli florets.[Pubmed:30274889]
Ultrason Sonochem. 2019 Jan;50:289-301.
Postharvest treatments such as wounding, ultrasound (US) and the exogenous application of ethylene (ET) and methyl jasmonate (MJ) have been studied as an effective tool to improve the content of secondary metabolites in fresh produce. The present study evaluated the immediate and late response (storage for 72h at 15 degrees C) to US treatment (20min, frequency 24kHz, amplitude 100mum) alone and combined with exogenous MJ (250ppm) and/or ET (1000ppm) on glucosinolates, isothiocyanates, phenolic compounds and ascorbic acid content in broccoli florets. US treatment increased the extractability of glucosinolates [glucoraphanin (795%), 4-hydroxy glucobrassicin (153%), glucobrassicin (78.6%)] and phenolics [1-sinapoyl-2-feruloylgentiobiose (57.23%)] as compared with the control (CT). The combined application of MJ and US in broccoli florets, induced a synergistic effect on the accumulation of 4-hydroxy glucobrassicin (187.1%), glucoerucin (111.92%), Gluconasturtiin (755.9%), neoglucobrassicin (232.8%), 3-O-caffeoylquinic acid (73.4%), 1-sinapoyl-2-ferulolylgentiobiose (56.0%), and 1,2,2-trisinapoylgentiobiose (136.7%) at 72h of storage. Interestingly, when the three stressors were applied together the synergistic effect of US+MJ observed on the accumulation of glucosinolates and phenolics was repressed. In general, the ascorbic acid content was not affected by US treatment and decreased in most samples during storage. However, when MJ+ET were applied, the content of total ascorbic acid was significantly reduced in CT+MJ+ET and US+MJ+ET samples after 72h of storage by 53.4% and 86.6%, respectively, as compared with CT 0h samples. Based on the results herein obtained, the application of US can be an effective tool to enhance the extractability of certain glucocosinolate and phenolic compounds in broccoli. Moreover, due to the synergistic effect observed on the accumulation of bioactive compounds, the combined application of US and MJ could be a practical approach to yield higher levels of glucosinolates and phenolic compounds in broccoli during storage.
The Role of the Glucosinolate-Myrosinase System in Mediating Greater Resistance of Barbarea verna than B. vulgaris to Mamestra brassicae Larvae.[Pubmed:30218254]
J Chem Ecol. 2018 Dec;44(12):1190-1205.
We investigated the influences of two structurally similar glucosinolates, phenethylglucosinolate (Gluconasturtiin, NAS) and its (S)-2-hydroxyl derivative glucobarbarin (BAR), as well as their hydrolysis products on larvae of the generalist Mamestra brassicae (Lepidoptera: Noctuidae). Previous results suggested a higher defensive activity of BAR than NAS based on resistance toward M. brassicae larvae of natural plant genotypes of Barbarea vulgaris R. Br. (Brassicaceae) dominated by BAR. In the present study, the hypothesis of a higher defensive activity of BAR than NAS was tested by comparing two Barbarea species similarly dominated either by BAR or by NAS and by testing effects of isolated BAR and NAS on larval survival and feeding preferences. Larvae reared on leaf disks of B. verna (Mill.) Asch. had a lower survival than those reared on B. vulgaris P- and G-chemotypes. Leaves of B. verna were dominated by NAS, whereas B. vulgaris chemotypes were dominated by BAR or its epimer. In addition, B. verna leaves showed a threefold higher activity of the glucosinolate-activating myrosinase enzymes. The main product of NAS from breakdown by endogenous enzymes including myrosinases ("autolysis") in B. verna leaves was phenethyl isothiocyanate, while the main products of BAR in autolyzed B. vulgaris leaves were a cyclized isothiocyanate product, namely an oxazolidine-2-thione, and a downstream metabolite, an oxazolidin-2-one. The glucosinolates BAR and NAS were isolated and offered to larvae on disks of cabbage. Both glucosinolates exerted similar negative effects on larval survival but effects of NAS tended to be more detrimental. Low concentrations of BAR, but not of NAS, stimulated larval feeding, whereas high BAR concentrations acted deterrent. NAS only tended to be deterrent at the highest concentration, but the difference was not significant. Recoveries of NAS and BAR on cabbage leaf disks were similar, and when hydrolyzed by mechanical leaf damage, the same isothiocyanate-type products as in Barbarea plants were formed with further conversion of BAR to cyclic products, (R)-5-phenyloxazolidine-2-thione [(R)-barbarin] and (R)-5-phenyloxazolidin-2-one [(R)-resedine]. We conclude that a previously proposed generally higher defensive activity of BAR than NAS to M. brassicae larvae could not be confirmed. Indeed, the higher resistance of NAS-containing B. verna plants may be due to a combined effect of rather high concentrations of NAS and a relatively high myrosinase activity or other plant traits not investigated yet.
Variation of glucosinolates on position orders of flower buds in turnip rape (Brassica rapa).[Pubmed:30174493]
Saudi J Biol Sci. 2017 Nov;24(7):1562-1566.
To glucosinolate (GSL) contents on flower buds depending on their position orders in turnip rape (Brassica rapa), three Japanese 'Nabana' cultivars such as cv. No. 21 (Brassica rapa, early type), cv. Husanohana (B. rapa, late type) and cv. Norin No. 20 (B. napus) were investigated using HPLC analysis. Ten GSLs including glucoraphanin, sinigrin, glucoalyssin, napoleiferin, gluconapin, 4-hydroxyglucobrassicin, glucobrassicanapin, glucobrassicin, and Gluconasturtiin were detected. Differences in individual and total GSL contents were found between two plant varieties, which are also depending on various developmental stages. Among the GSLs, gluconapin (mean 23.11 mumole/g dry weight (DW) and glucobrassicanapin (mean 13.41 mumole/g DW) documented the most abundant compounds and contributed average 39 and 27% of the total GSLs, but indolyl and aromatic GSLs together accounted >10% of the total GSLs. The presence of significant quantities of gluconapin in the cultivars should be studied more extensively, since the GSL is mainly responsible for the bitter taste.
Influence of silver nanoparticles on the enhancement and transcriptional changes of glucosinolates and phenolic compounds in genetically transformed root cultures of Brassica rapa ssp. rapa.[Pubmed:30056602]
Bioprocess Biosyst Eng. 2018 Nov;41(11):1665-1677.
Glucosinolates (GSLs) and phenolic compounds (PCs) are biologically active and involved in the defense reaction of plants; these compounds have a beneficial effect on human health. In this study, we described the influence of biologically synthesized silver nanoparticles (Ag NPs) to enhance the phytochemicals (GSLs and PCs), their transcription levels, and their biological activities in genetically transformed root cultures (hairy root cultures) of Brassica rapa. The concentrations of silver and reactive oxygen species (malondialdehyde and hydrogen peroxide) were highly elevated in the Ag NP-elicited hairy roots (HRs). Glucosinolates (glucoallysin, glucobrassicanapin, sinigrin, progoitrin, gluconapin, 4-methoxyglucobrassicin, 4-hydroxyglucobrassicin, glucobrassicin, neoglucobrassicin, and Gluconasturtiin) and their transcripts (MYB34, MYB51, MYB28, and MYB29) were significantly enhanced in the Ag NP-elicited HRs. Moreover, the phenolic compounds (flavonols, hydroxybenzoic, and hydroxycinnamic acids) were significantly enriched in the Ag NP-elicited HRs. Total phenolic and flavonoid concentrations and their transcripts (PAL, CHI, and FLS) were higher in the Ag NP-elicited HRs than in the non-elicited HRs. Additionally, biological (antioxidant, antimicrobial, and anticancer) activities were significantly higher in the Ag NP-elicited HRs than in the non-elicited HRs. The Ag NP-elicited HR cultures offered an efficient and promising in vitro method to increase the production of health-promoting bioactive compounds, which may be useful in nutraceutical and pharmaceutical industries.
Phenethyl Isothiocyanate, a Dual Activator of Transcription Factors NRF2 and HSF1.[Pubmed:29710398]
Mol Nutr Food Res. 2018 Sep;62(18):e1700908.
Cruciferous vegetables are rich sources of glucosinolates which are the biogenic precursor molecules of isothiocyanates (ITCs). The relationship between the consumption of cruciferous vegetables and chemoprotection has been widely documented in epidemiological studies. Phenethyl isothiocyanate (PEITC) occurs as its glucosinolate precursor Gluconasturtiin in the cruciferous vegetable watercress (Nasturtium officinale). PEITC has multiple biological effects, including activation of cytoprotective pathways, such as those mediated by the transcription factor nuclear factor erythroid 2 p45-related factor 2 (NRF2) and the transcription factor heat shock factor 1 (HSF1), and can cause changes in the epigenome. However, at high concentrations, PEITC leads to accumulation of reactive oxygen species and cytoskeletal changes, resulting in cytotoxicity. Underlying these activities is the sulfhydryl reactivity of PEITC with cysteine residues in its protein targets. This chemical reactivity highlights the critical importance of the dose of PEITC for achieving on-target selectivity, which should be carefully considered in the design of future clinical trials.
Exposure of kale root to NaCl and Na2SeO3 increases isothiocyanate levels and Nrf2 signalling without reducing plant root growth.[Pubmed:29507323]
Sci Rep. 2018 Mar 5;8(1):3999.
A plant factory is a closed cultivation system that provides a consistent and modified environment for plant growth. We speculated that treatment of kale (Brassica oleracea) grown in a plant factory with NaCl, Na2SeO3, or both would increase the bioactive phytochemical levels including glucosinolates (GLSs) and isothiocyanates (ITCs), the key molecules in cancer prevention. The kale was harvested and analysed after treatment with NaCl and Na2SeO3 alone or in combination for 1 or 2 weeks. Exposure to NaCl alone but not Na2SeO3 increased plant root growth. Levels of sinigrin were increased by a 2-week exposure to Na2SeO3 alone or in combination with NaCl, whereas no changes were observed in glucoraphanin and Gluconasturtiin Gluconasturtiin levels. Importantly, the ITC concentration was affected by 2-week treatment with both compounds. To evaluate the bioactivity of kale, HepG2 human hepatoma cells were treated with plant extract for 6 h. Only the extract of kale roots exposed to a combination NaCl and Na2SeO3 for 2 weeks showed an increased expression of nuclear factor erythroid 2-related factor (Nrf2), which regulates genes encoding antioxidant proteins. These data suggest that co-treatment with NaCl and Na2SeO3 increased the ITC content and chemopreventive effects of kale root.
Molecular characterization of glucosinolates and carotenoid biosynthetic genes in Chinese cabbage (Brassica rapa L. ssp. pekinensis).[Pubmed:29379360]
Saudi J Biol Sci. 2018 Jan;25(1):71-82.
The present study aimed to investigate the contents of glucosinolates (GSLs) and carotenoids in eleven varieties of Chinese cabbage in relation to the expression level of the important transcription factors. MS and HPLC analysis identified the presence of 13 GSLs (progoitrin, sinigrin, glucoalyssin, gluconapoleiferin, gluconapin, glucocochlearin, glucobrassicanapin, glucoerucin, 4-hydroxyglucobrassicin, glucobrassicin, 4-methoxyglucobrassicin, neoglucobrassicin and Gluconasturtiin) and four carotenoids (lutein, zeaxanthin, alpha-carotene and beta-carotene). GSL contents were varied among the different cabbage varieties. The total GSL content ranged from 2.7 to 57.88 mumol/g DW. The proportion of gluconapin (54%) and glucobrassicanapin (22%) was higher in all the varieties, respectively. Results documented the variation in total and individual carotenoid contents that have also been observed among different varieties; however, the total carotenoid contents ranged from 289.12 to 1001.41 mg kg(-1) DW (mean 467.66). Interestingly, the proportion of lutein (66.5) and beta-carotene (25.9) were higher than alpha-carotene (5.1) and zeaxanthin (2.5%). Consequently, the expression level of the regulatory gene, MYB28 was higher in 'K0648' and was directly proportional to GSL content. Similarly, the expression levels of 1-PSY were higher in 'K0112'; however, the expression levels of 2-ZDS, 3-LCYB, 4-LCYE, 5-CHXB and 7-NCED genes showed no significant difference. In addition, the correlation between GSL and carotenoid contents and gene expression level showed moderate significant difference in each Chinese cabbage.


