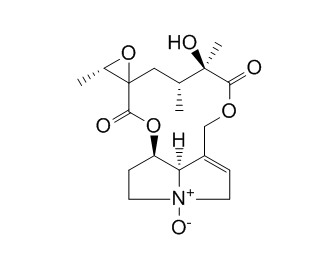
Jacobine N-oxideCAS# 38710-25-7 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 38710-25-7 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | White powder |
| Formula | C18H25NO7 | M.Wt | 367.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in methanol and water | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Jacobine N-oxide Dilution Calculator

Jacobine N-oxide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7218 mL | 13.6091 mL | 27.2183 mL | 54.4366 mL | 68.0457 mL |
| 5 mM | 0.5444 mL | 2.7218 mL | 5.4437 mL | 10.8873 mL | 13.6091 mL |
| 10 mM | 0.2722 mL | 1.3609 mL | 2.7218 mL | 5.4437 mL | 6.8046 mL |
| 50 mM | 0.0544 mL | 0.2722 mL | 0.5444 mL | 1.0887 mL | 1.3609 mL |
| 100 mM | 0.0272 mL | 0.1361 mL | 0.2722 mL | 0.5444 mL | 0.6805 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Intermedine N-oxide
Catalog No.:BCN8931
CAS No.:95462-14-9
- Integerrimine N-oxide
Catalog No.:BCN8930
CAS No.:85955-28-8
- Heliotridine
Catalog No.:BCN8929
CAS No.:520-63-8
- Erucifolin N-oxide
Catalog No.:BCN8928
CAS No.:123864-94-8
- 19-O-beta-D-carboxyglucopyranosyl-12-O-beta-D-glucopyranosyl-11,16-dihydroxyabieta-8,11,13-triene
Catalog No.:BCN8926
CAS No.:1011714-20-7
- Nardoaristolone B
Catalog No.:BCN8925
CAS No.:1422517-82-5
- (3R,5S,E)-1,7-Diphenylhept-1-ene-3,5-diol
Catalog No.:BCN8924
CAS No.:232261-31-3
- (3S,5S,E)-1,7-Diphenylhept-1-ene-3,5-diol
Catalog No.:BCN8923
CAS No.:87095-75-8
- 5-Hydroxy-7,8-dimethoxy (2R)-flavanone-5-O-beta-D-glucopyranoside
Catalog No.:BCN8922
CAS No.:942626-74-6
- (1E)-3-methoxy-8,12-epoxygermacra-1,7,10,11-tetraen-6-one
Catalog No.:BCN8921
CAS No.:1393342-06-7
- Ginsenoside MC
Catalog No.:BCN8920
CAS No.:175484-06-7
- 3-Hydroxy-4',5-dimethoxystilbene
Catalog No.:BCN8919
CAS No.:58436-29-6
- Merenskine
Catalog No.:BCN8933
CAS No.:96657-94-2
- Merenskine N-oxide
Catalog No.:BCN8934
CAS No.:96657-95-3
- 7-O-Acetyllycopsamine N-oxide
Catalog No.:BCN8935
CAS No.:685132-58-5
- Riddelline N-oxide
Catalog No.:BCN8936
CAS No.:75056-11-0
- Senecivernine N-oxide
Catalog No.:BCN8937
CAS No.:101687-28-9
- Sceleratine N-oxide
Catalog No.:BCN8938
CAS No.:103184-92-5
- Glucohesperin
Catalog No.:BCN8939
CAS No.:33049-17-1
- 6-Hydroxytropinone
Catalog No.:BCN8940
CAS No.:5932-53-6
- Glucoraphenin
Catalog No.:BCN8942
CAS No.:108844-81-1
- Heliosupine N-oxide
Catalog No.:BCN8943
CAS No.:31701-88-9
- Indicine hydrochloride
Catalog No.:BCN8945
CAS No.:1195140-94-3
- Merepoxine
Catalog No.:BCN8946
CAS No.:115777-94-1
The effect of structurally related metabolites on insect herbivores: A case study on pyrrolizidine alkaloids and western flower thrips.[Pubmed:28267991]
Phytochemistry. 2017 Jun;138:93-103.
Plant specialised metabolites (SMs) are very diverse in terms of both their number and chemical structures with more than 200,000 estimated compounds. This chemical diversity occurs not only among different groups of compounds but also within the groups themselves. In the context of plant-insect interactions, the chemical diversity within a class of structurally related metabolites is generally also related to their bioactivity. In this study, we tested firstly whether individual SMs within the group of pyrrolizidine alkaloids (PAs) differ in their effects on insect herbivores (western flower thrips, Frankliniella occidentalis). Secondly, we tested combinations of PA N-oxides to determine whether they are more active than their individual components. We also evaluated the bioactivity of six PA free bases and their corresponding N-oxides. At concentrations similar to that in plants, several PAs reduced thrip's survival but the effect also differed strongly among PAs. In general, PA free bases caused a lower survival than their corresponding N-oxides. Among the tested PA free bases, we found jacobine and retrorsine to be the most active against second instar larvae of thrips, followed by erucifoline and seneciphylline, while senecionine and monocrotaline did not exhibit significant dose-dependent effects on thrip's survival. In the case of PA N-oxides, we found that only senecionine N-oxide and Jacobine N-oxide reduced thrip's survival, although the effect of senecionine N-oxide was weak. Combinations of PA N-oxides showed no synergistic effects. These findings indicate the differences observed in the effect of structurally related SMs on insect herbivores. It is of limited value to study the bioactivity of combined groups, such as PAs, without taking their composition into account.


