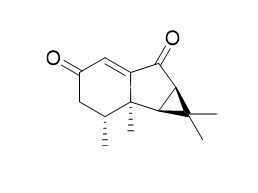
Nardoaristolone BCAS# 1422517-82-5 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 1422517-82-5 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C14H18O2 | M.Wt | 218.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nardoaristolone B, a nor-sesquiterpenoid with an unusual fused ring system and having protective effects on the injury of neonatal rat cardiomyocytes. The novel mosquito-repellentsynthetic hydrindanesbased on noreremophilanes and nardoaristolone B which show increasedactivity against adult females of Aedes aegypti. |
| In vitro | Isolation of Novel Sesquiterpeniods and Anti-neuroinflammatory Metabolites from Nardostachys jatamansi.[Pubmed: 30227591]Molecules. 2018 Sep 17;23(9). pii: E2367.Nardostachys jatamansi contains various types of sesquiterpenoids that may play an important role in the potency of plant's anti-inflammatory effects, depending on their structure.
|
| Structure Identification | Org Lett. 2014 Aug 15;16(16):4252-5.Total synthesis of (±)-nardoaristolone B and its analogues.[Pubmed: 25080212]The first total synthesis of Nardoaristolone B, a nor-sesquiterpenoid with an unusual fused ring system and having protective effects on the injury of neonatal rat cardiomyocytes, has been accomplished.
Org Lett. 2013 Mar 1;15(5):1000-3.Nardoaristolones A and B, two terpenoids with unusual skeletons from Nardostachys chinensis Batal.[Pubmed: 23394111]
ACS Omega, 2019, 4(1):2188-2195.Insect-Repellent and Mosquitocidal Effects of Noreremophilane- and Nardoaristolone-Based Compounds[Reference: WebLink]
|

Nardoaristolone B Dilution Calculator

Nardoaristolone B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5809 mL | 22.9043 mL | 45.8085 mL | 91.617 mL | 114.5213 mL |
| 5 mM | 0.9162 mL | 4.5809 mL | 9.1617 mL | 18.3234 mL | 22.9043 mL |
| 10 mM | 0.4581 mL | 2.2904 mL | 4.5809 mL | 9.1617 mL | 11.4521 mL |
| 50 mM | 0.0916 mL | 0.4581 mL | 0.9162 mL | 1.8323 mL | 2.2904 mL |
| 100 mM | 0.0458 mL | 0.229 mL | 0.4581 mL | 0.9162 mL | 1.1452 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (3R,5S,E)-1,7-Diphenylhept-1-ene-3,5-diol
Catalog No.:BCN8924
CAS No.:232261-31-3
- (3S,5S,E)-1,7-Diphenylhept-1-ene-3,5-diol
Catalog No.:BCN8923
CAS No.:87095-75-8
- 5-Hydroxy-7,8-dimethoxy (2R)-flavanone-5-O-beta-D-glucopyranoside
Catalog No.:BCN8922
CAS No.:942626-74-6
- (1E)-3-methoxy-8,12-epoxygermacra-1,7,10,11-tetraen-6-one
Catalog No.:BCN8921
CAS No.:1393342-06-7
- Ginsenoside MC
Catalog No.:BCN8920
CAS No.:175484-06-7
- 3-Hydroxy-4',5-dimethoxystilbene
Catalog No.:BCN8919
CAS No.:58436-29-6
- Isoeuphorbetin
Catalog No.:BCN8918
CAS No.:50677-55-9
- Licoflavanone
Catalog No.:BCN8917
CAS No.:119240-82-3
- Schisphenin E
Catalog No.:BCN8916
CAS No.:1311376-52-9
- Stevia impurity (13-[(2-O-6-deoxy-β-D-glucopyranosyl-3-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy]ent-kaur-16-en-19-oic acid β-D-glucopyranosyl ester)
Catalog No.:BCN8915
CAS No.:1309929-72-3
- Methylgomisin O
Catalog No.:BCN8914
CAS No.:1276654-07-9
- Dichotomine B
Catalog No.:BCN8913
CAS No.:755036-41-0
- 19-O-beta-D-carboxyglucopyranosyl-12-O-beta-D-glucopyranosyl-11,16-dihydroxyabieta-8,11,13-triene
Catalog No.:BCN8926
CAS No.:1011714-20-7
- Erucifolin N-oxide
Catalog No.:BCN8928
CAS No.:123864-94-8
- Heliotridine
Catalog No.:BCN8929
CAS No.:520-63-8
- Integerrimine N-oxide
Catalog No.:BCN8930
CAS No.:85955-28-8
- Intermedine N-oxide
Catalog No.:BCN8931
CAS No.:95462-14-9
- Jacobine N-oxide
Catalog No.:BCN8932
CAS No.:38710-25-7
- Merenskine
Catalog No.:BCN8933
CAS No.:96657-94-2
- Merenskine N-oxide
Catalog No.:BCN8934
CAS No.:96657-95-3
- 7-O-Acetyllycopsamine N-oxide
Catalog No.:BCN8935
CAS No.:685132-58-5
- Riddelline N-oxide
Catalog No.:BCN8936
CAS No.:75056-11-0
- Senecivernine N-oxide
Catalog No.:BCN8937
CAS No.:101687-28-9
- Sceleratine N-oxide
Catalog No.:BCN8938
CAS No.:103184-92-5
Isolation of Novel Sesquiterpeniods and Anti-neuroinflammatory Metabolites from Nardostachys jatamansi.[Pubmed:30227591]
Molecules. 2018 Sep 17;23(9). pii: molecules23092367.
Nardostachys jatamansi contains various types of sesquiterpenoids that may play an important role in the potency of plant's anti-inflammatory effects, depending on their structure. In this study, five new sesquiterpenoids, namely kanshone L (1), kanshone M (2), 7-methoxydesoxo-narchinol (3), kanshone N (4), and nardosdaucanol (5), were isolated along with four known terpenoids (kanshone D (6), nardosinanone G (7), narchinol A (8), and Nardoaristolone B (9)) from the rhizomes and roots of Nardostachys jatamansi. Their structures were determined by analyzing 1D and 2D NMR and MS data. Among the nine sesquiterpenoids, compounds 3, 4, and 8 were shown to possess dose-dependent inhibitory effects against lipopolysaccharide (LPS)-stimulated nitric oxide (NO) production in BV2 microglial cells. Furthermore, compounds 3, 4, and 8 exhibited anti-neuroinflammatory effects by inhibiting the production of pro-inflammatory mediators, including prostaglandin E(2) (PGE(2)), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) proteins, as well as pro-inflammatory cytokines, such as interleukin (IL)-1beta, IL-12 and tumor necrosis factor-alpha (TNF-alpha), in LPS-stimulated BV2 microglial cells. Moreover, these compounds were shown to inhibit the activation of the NF-kappaB signaling pathway in LPS-stimulated BV2 microglial cells by suppressing the phosphorylation of IkappaB-alpha and blocking NF-kappaB translocation. In conclusion, five new and four known sesquiterpenoids were isolated from Nardostachys jatamansi, and compounds 3, 4, and 8 exhibited anti-neuroinflammatory effects in LPS-stimulated BV2 microglial cells through inhibiting of NF-kappaB signaling pathway.
Enantioselective total synthesis of (-)-nardoaristolone B via a gold(I)-catalyzed oxidative cyclization.[Pubmed:25563976]
Org Lett. 2015 Feb 6;17(3):461-3.
The first enantioselective total synthesis of (-)-Nardoaristolone B is accomplished by the implementation of an enantio- and diastereoselective copper(I)-catalyzed conjugate addition/enolate trapping sequence and a gold(I)-catalyzed oxidative cyclization (intermolecular oxidant), employed for the first time in total synthesis.
Total synthesis of (+/-)-nardoaristolone B and its analogues.[Pubmed:25080212]
Org Lett. 2014 Aug 15;16(16):4252-5.
The first total synthesis of Nardoaristolone B, a nor-sesquiterpenoid with an unusual fused ring system and having protective effects on the injury of neonatal rat cardiomyocytes, has been accomplished. Stereoselective synthesis of its novel analogues inlcuding exo-cyclopropyl ring fusion is also part of this disclosure. In addition, an alternate and more efficient one-step method to make a 3/5/6 tricyclic ring system using the Robinson annulation method has been developed toward the generation of a library of compounds around this skeleton.
Nardoaristolones A and B, two terpenoids with unusual skeletons from Nardostachys chinensis Batal.[Pubmed:23394111]
Org Lett. 2013 Mar 1;15(5):1000-3.
Nardoaristolones A and B, two novel terpenoids derived from the aristolane-type sesquiterpenoid, were isolated from the underground parts of Nardostachys chinensis Batal. Nardoaristolone A is the first reported aristolane-chalcone derivative, while Nardoaristolone B possesses a nor-aristolane sesquiterpenoid skeleton with an unusual 3/5/6 tricyclic ring system. Their structures were elucidated by spectroscopic measurements, and the absolute configurations were established by single-crystal X-ray diffraction experiments.


