LicoflavanoneCAS# 119240-82-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 119240-82-3 | SDF | Download SDF |
| PubChem ID | 14218027 | Appearance | Powder |
| Formula | C20H20O5 | M.Wt | 340.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
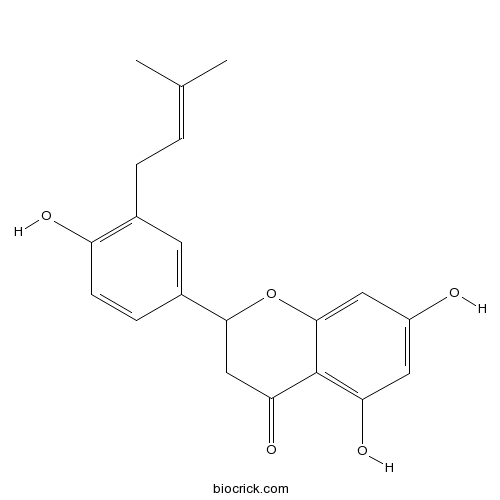
| Chemical Name | 5,7-dihydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)phenyl]-2,3-dihydrochromen-4-one | ||
| SMILES | CC(=CCC1=C(C=CC(=C1)C2CC(=O)C3=C(C=C(C=C3O2)O)O)O)C | ||
| Standard InChIKey | CGKWSLSAYABZTL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H20O5/c1-11(2)3-4-12-7-13(5-6-15(12)22)18-10-17(24)20-16(23)8-14(21)9-19(20)25-18/h3,5-9,18,21-23H,4,10H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Licoflavanone exhibits antioxidant and anti-inflammatory activities,it markedly decreases pro-inflammatory cytokines and cyclooxygenase 2/inducible nitric oxide synthase (COX-2/iNOS) expression levels. |
| Targets | COX | NOS | NF-kB | MAPK |
| In vitro | Antioxidant and Anti-Inflammatory Activities of Flavanones from Glycyrrhiza glabra L. (licorice) Leaf Phytocomplexes: Identification of Licoflavanone as a Modulator of NF-kB/MAPK Pathway.[Pubmed: 31226797]Antioxidants (Basel). 2019 Jun 20;8(6). pii: E186.Inflammation represents an adaptive response generated by injuries or harmful stimuli. Natural remedies represent an interesting alternative to traditional therapies, involving several biochemical pathways. Besides, the valorization of agrochemical wastes nowadays seems to be a feasible way to reduce the health spending and improve the accessibility at bioactive natural compounds.
|
| Structure Identification | Phytochemistry (Oxford), 1996, 42(3):701-704.Flavonoid variation in the leaves of Glycyrrhiza glabra.[Reference: WebLink]
|

Licoflavanone Dilution Calculator

Licoflavanone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9377 mL | 14.6886 mL | 29.3772 mL | 58.7544 mL | 73.443 mL |
| 5 mM | 0.5875 mL | 2.9377 mL | 5.8754 mL | 11.7509 mL | 14.6886 mL |
| 10 mM | 0.2938 mL | 1.4689 mL | 2.9377 mL | 5.8754 mL | 7.3443 mL |
| 50 mM | 0.0588 mL | 0.2938 mL | 0.5875 mL | 1.1751 mL | 1.4689 mL |
| 100 mM | 0.0294 mL | 0.1469 mL | 0.2938 mL | 0.5875 mL | 0.7344 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Schisphenin E
Catalog No.:BCN8916
CAS No.:1311376-52-9
- Stevia impurity (13-[(2-O-6-deoxy-β-D-glucopyranosyl-3-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy]ent-kaur-16-en-19-oic acid β-D-glucopyranosyl ester)
Catalog No.:BCN8915
CAS No.:1309929-72-3
- Methylgomisin O
Catalog No.:BCN8914
CAS No.:1276654-07-9
- Dichotomine B
Catalog No.:BCN8913
CAS No.:755036-41-0
- 2-Methoxy-5-acetoxy-furanogermacr-1(10)-en-6-one
Catalog No.:BCN8912
CAS No.:1809980-25-3
- Methyl neochebulinate
Catalog No.:BCN8911
CAS No.:1236310-34-1
- Biatractylolide
Catalog No.:BCN8910
CAS No.:182426-37-5
- Arisantetralone B
Catalog No.:BCN8909
CAS No.:1161947-96-1
- Rebaudioside F
Catalog No.:BCN8908
CAS No.:438045-89-7
- Arisanschinin E
Catalog No.:BCN8907
CAS No.:1333378-33-8
- Isolappaol C
Catalog No.:BCN8906
CAS No.:929905-15-7
- Stevioside D
Catalog No.:BCN8905
CAS No.:1310055-59-4
- Isoeuphorbetin
Catalog No.:BCN8918
CAS No.:50677-55-9
- 3-Hydroxy-4',5-dimethoxystilbene
Catalog No.:BCN8919
CAS No.:58436-29-6
- Ginsenoside MC
Catalog No.:BCN8920
CAS No.:175484-06-7
- (1E)-3-methoxy-8,12-epoxygermacra-1,7,10,11-tetraen-6-one
Catalog No.:BCN8921
CAS No.:1393342-06-7
- 5-Hydroxy-7,8-dimethoxy (2R)-flavanone-5-O-beta-D-glucopyranoside
Catalog No.:BCN8922
CAS No.:942626-74-6
- (3S,5S,E)-1,7-Diphenylhept-1-ene-3,5-diol
Catalog No.:BCN8923
CAS No.:87095-75-8
- (3R,5S,E)-1,7-Diphenylhept-1-ene-3,5-diol
Catalog No.:BCN8924
CAS No.:232261-31-3
- Nardoaristolone B
Catalog No.:BCN8925
CAS No.:1422517-82-5
- 19-O-beta-D-carboxyglucopyranosyl-12-O-beta-D-glucopyranosyl-11,16-dihydroxyabieta-8,11,13-triene
Catalog No.:BCN8926
CAS No.:1011714-20-7
- Erucifolin N-oxide
Catalog No.:BCN8928
CAS No.:123864-94-8
- Heliotridine
Catalog No.:BCN8929
CAS No.:520-63-8
- Integerrimine N-oxide
Catalog No.:BCN8930
CAS No.:85955-28-8
Antioxidant and Anti-Inflammatory Activities of Flavanones from Glycyrrhiza glabra L. (licorice) Leaf Phytocomplexes: Identification of Licoflavanone as a Modulator of NF-kB/MAPK Pathway.[Pubmed:31226797]
Antioxidants (Basel). 2019 Jun 20;8(6). pii: antiox8060186.
Inflammation represents an adaptive response generated by injuries or harmful stimuli. Natural remedies represent an interesting alternative to traditional therapies, involving several biochemical pathways. Besides, the valorization of agrochemical wastes nowadays seems to be a feasible way to reduce the health spending and improve the accessibility at bioactive natural compounds. In this context, the chemical composition of three Glycyrrhiza glabra L. (licorice) leaf extracts, obtained through maceration or ultrasound-assisted method (fresh and dried leaves) was investigated. A guided fractionation obtained three main components: pinocembrin, glabranin and Licoflavanone. All the extracts showed similar antioxidant properties, evaluated by 2,2'-diphenyl-1-picrylhydrazyl (DPPH) or 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) Diammonium Salt (ABTS) assay, while, among the isolated compounds, Licoflavanone exhibited the best antioxidant activity. The anti-inflammatory activity of the extracts and the purified compounds was investigated in lipopolysaccharide (LPS)-stimulated RAW 264.7 murine macrophages. Extract C and Licoflavanone showed a good anti-inflammatory activity without affecting cell viability, as they decreased nitrite levels even when used at 12.5 mug/mL (p < 0.005) and 50 muM concentration (p < 0.001), respectively. Interestingly, Licoflavanone markedly decreased pro-inflammatory cytokines and cyclooxygenase 2/inducible nitric oxide synthase (COX-2/iNOS) expression levels (p < 0.001). A modulation of nuclear factor kappa B/mitogen-activated protein kinases (NF-kB/MAPK) pathway underlay such behavior, highlighting the potential of this natural compound as a new scaffold in anti-inflammatory drug research.
The anticancer potential of flavonoids isolated from the stem bark of Erythrina suberosa through induction of apoptosis and inhibition of STAT signaling pathway in human leukemia HL-60 cells.[Pubmed:23850732]
Chem Biol Interact. 2013 Sep 25;205(2):128-37.
Erythrina suberosa is an ornamental tall tree found in India, Pakistan, Nepal, Bhutan, Burma, Thailand and Vietnam. We have isolated four known distinct metabolites designated as alpha-Hydroxyerysotrine, 4'-Methoxy Licoflavanone (MLF), Alpinumisoflavone (AIF) and Wighteone. Among the four isolated metabolites the two flavonoids, MLF and AIF were found to be the most potent cytotoxic agent with IC50 of approximately 20muM in human leukemia HL-60 cells. We are reporting first time the anticancer and apoptotic potential of MLF and AIF in HL-60 cells. Both MLF and AIF inhibited HL-60 cell proliferation and induce apoptosis as measured by several biological endpoints. MLF and AIF induce apoptosis bodies formation, enhanced annexinV-FITC binding of the cells, increased sub-G0 cell fraction, loss of mitochondrial membrane potential (Deltapsim), release of cytochrome c, Bax, activation of caspase-9, caspase-3 and PARP (poly ADP Ribose polymers) cleavage in HL-60 cells. MLF and AIF also increase the expression of apical death receptor, Fas, with inhibition of anti-apoptotic protein Bid. All the above parameters revealed that these two flavonoids induce apoptosis through both extrinsic and intrinsic apoptotic pathways in HL-60 cells. In spite of apoptosis, these two flavonoids significantly inhibit nuclear transcription factor NF-kappaB and STAT (Signal Transducer and Activator of Transcription) signaling pathway, which are highly expressed in leukemia. The present study provide an insight of molecular mechanism of cell death induced by MLF and AIF in HL-60 cells which may be useful in managing and treating leukemia.
Phenolics from Glycyrrhiza glabra roots and their PPAR-gamma ligand-binding activity.[Pubmed:20022509]
Bioorg Med Chem. 2010 Jan 15;18(2):962-70.
Bioassay-guided fractionation of the EtOH extract of licorice (Glycyrrhiza glabra roots), using a GAL-4-PPAR-gamma chimera assay method, resulted in the isolation of 39 phenolics, including 10 new compounds (1-10). The structures of the new compounds were determined by analysis of their spectroscopic data. Among the isolated compounds, 5'-formylglabridin (5), (2R,3R)-3,4',7-trihydroxy-3'-prenylflavane (7), echinatin, (3R)-2',3',7-trihydroxy-4'-methoxyisoflavan, kanzonol X, kanzonol W, shinpterocarpin, Licoflavanone A, glabrol, shinflavanone, gancaonin L, and glabrone all exhibited significant PPAR-gamma ligand-binding activity. The activity of these compounds at a sample concentration of 10microg/mL was three times more potent than that of 0.5microM troglitazone.
Prenylated flavonoids with PTP1B inhibitory activity from the root bark of Erythrina mildbraedii.[Pubmed:18175982]
Chem Pharm Bull (Tokyo). 2008 Jan;56(1):85-8.
Phytochemical study on an EtOAc-soluble extract of the root bark of Erythrina mildbraedii resulted in the isolation of six prenylated flavonoids 1-6. Based on physicochemical and spectroscopic analyses, their structures were determined to be new natural products Licoflavanone-4'-O-methyl ether (1), 2',7-dihydroxy-4'-methoxy-5'-(3-methylbut-2-enyl)isoflavone (2), and (3R)-2',7-dihydroxy-3'-(3-methylbut-2-enyl)-2''',2'''-dimethylpyrano[5''',6''' :4',5']isoflavan (3), along with three known compounds erythrinin B (4), abyssinin II (5), and parvisoflavone B (6). All the isolates, except for compound 4, inhibited PTP1B activity in vitro with IC(50) values ranging from 5.3 to 42.6 microM. This result further suggests that the prenyl group on the B ring of flavonoids plays an important role in suppressing the enzyme PTP1B.


