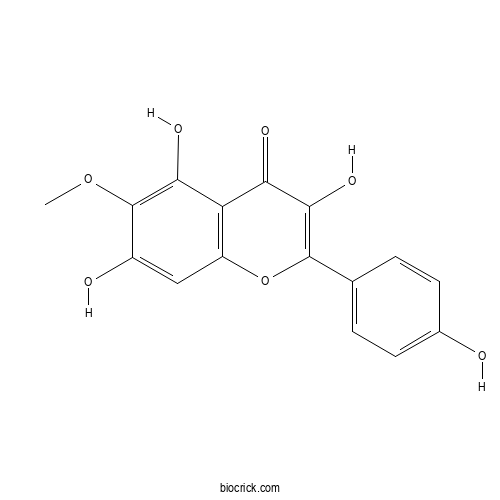
6-MethoxykaempferolCAS# 32520-55-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 32520-55-1 | SDF | Download SDF |
| PubChem ID | 5377945 | Appearance | Yellow powder |
| Formula | C16H12O7 | M.Wt | 316.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-6-methoxychromen-4-one | ||
| SMILES | COC1=C(C2=C(C=C1O)OC(=C(C2=O)O)C3=CC=C(C=C3)O)O | ||
| Standard InChIKey | OGQSUSFDBWGFFJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12O7/c1-22-16-9(18)6-10-11(13(16)20)12(19)14(21)15(23-10)7-2-4-8(17)5-3-7/h2-6,17-18,20-21H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

6-Methoxykaempferol Dilution Calculator

6-Methoxykaempferol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1616 mL | 15.8078 mL | 31.6156 mL | 63.2311 mL | 79.0389 mL |
| 5 mM | 0.6323 mL | 3.1616 mL | 6.3231 mL | 12.6462 mL | 15.8078 mL |
| 10 mM | 0.3162 mL | 1.5808 mL | 3.1616 mL | 6.3231 mL | 7.9039 mL |
| 50 mM | 0.0632 mL | 0.3162 mL | 0.6323 mL | 1.2646 mL | 1.5808 mL |
| 100 mM | 0.0316 mL | 0.1581 mL | 0.3162 mL | 0.6323 mL | 0.7904 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,6-Dihydroxy-3,7,4'-trimethoxyflavone
Catalog No.:BCN0671
CAS No.:84019-17-0
- 5,6-Dihydroxy-3,7,3',4'-tetramethoxyflavone
Catalog No.:BCN0670
CAS No.:63296-15-1
- 3,5,8,3',4',5'-Hexamethoxy-6,7-methylenedioxyflavone
Catalog No.:BCN0669
CAS No.:82668-95-9
- 7,4'-Dihydroxy-6,8-diprenylflavanone
Catalog No.:BCN0668
CAS No.:50939-03-2
- Isosativanone
Catalog No.:BCN0667
CAS No.:82829-55-8
- Hydnocarpin
Catalog No.:BCN0666
CAS No.:51419-48-8
- Kaempferol 3-O-(3",6"-di-O-E-p-coumaroyl)-β-D-glucopyranoside
Catalog No.:BCN0665
CAS No.:218605-31-3
- 6-Feruloylcatalpol
Catalog No.:BCN0664
CAS No.:770721-33-0
- Multifloroside
Catalog No.:BCN0663
CAS No.:131836-10-7
- 10-Hydroxyoleoside dimethyl ester
Catalog No.:BCN0662
CAS No.:91679-27-5
- Jasnervoside G
Catalog No.:BCN0661
CAS No.:1622337-40-9
- Jaspolyanthoside
Catalog No.:BCN0660
CAS No.:175448-05-2
- 6-Methoxykaempferol 3-O-glucoside
Catalog No.:BCN0673
CAS No.:63422-27-5
- 2'-Hydroxyneophellamuretin
Catalog No.:BCN0674
CAS No.:1396769-20-2
- Nuchensin
Catalog No.:BCN0675
CAS No.:25782-24-5
- 5,8-Dihydroxy-6,7-dimethoxyflavone
Catalog No.:BCN0676
CAS No.:73202-52-5
- Volkensiflavone
Catalog No.:BCN0677
CAS No.:27542-37-6
- Quercitrin 2''-O-arabinoside
Catalog No.:BCN0678
CAS No.:104683-55-8
- Mikanin
Catalog No.:BCN0679
CAS No.:4324-53-2
- 6'-Prenylisorhamnetin
Catalog No.:BCN0680
CAS No.:1859979-02-4
- 2'-Prenylisorhamnetin
Catalog No.:BCN0681
CAS No.:1932668-04-6
- Orientin 2''-O-rhamnoside
Catalog No.:BCN0682
CAS No.:81398-30-3
- Naringenin 7-O-β-D-glucuronide methyl ester
Catalog No.:BCN0683
CAS No.:1985597-72-5
- Eriodictyol 7-O-β-D-glucuronide ethyl ester
Catalog No.:BCN0684
CAS No.:847025-48-3
Effect of extracts and isolated compounds derived from Retama monosperma (L.) Boiss. on anti-aging gene expression in human keratinocytes and antioxidant activity.[Pubmed:34314805]
J Ethnopharmacol. 2021 Nov 15;280:114451.
ETHNOPHARMACOLOGICAL RELEVANCE: Moroccan folk medicine treats skin cicatrization with Retama monosperma (L.) Boiss. locally named "Rtem", but the mechanism involved is still not well known. Traditional healers use the plant in small doses as an anthelmintic, disinfectant and an effective abortive. In addition, the cladodes powder mixed with honey is employed as purgative and vermifuge. Equally, the SIRT1 and SIRT3 genes activation and sirtuin proteins expression, which delay cellular senescence, participate in wound healing and skin regeneration especially, SIRT1 the most studied gene, leads to fast skin restoration and cicatrization. AIM OF THE STUDY: In this study, we evaluated the ability of the Retama monosperma (L.)Boiss. flowers and seeds extracts and the isolated compounds in augmenting the SIRT1 and SIRT3 gene expression in HaCaT cells and expressing the antioxidant activity. MATERIALS AND METHODS: We examined for quantitative expression levels of SIRT1 and SIRT3 in HaCaT cell by qRT-PCR and the antioxidant activity by four tests (conjugated diene, TBARS assay, DPPH scavenging activity and H2O2 radical scavenging assay) of diethyl ether extract of flowers (DEF extract) and ethyl acetate extract of seeds (EAS extract) of R. monosperma(L.) Boiss. and the isolated compounds (quercetin, 6-Methoxykaempferol, kaempferol and genistein). RESULTS: The screening system by EGFP fluorescence revealed that all samples and resveratrol significantly increase SIRT1 and SIRT3 promoters activities in HaCaT cells with p< 0.05. Furthermore, EAS, quercetin, 6-Methoxykaempferol and kaempferol increase significantly (p< 0.05) SIRT1 (3.43, 1.18, 2.62, and 1.72 expression quantity, respectively) and SIRT3 (16.27, 5.01, 3.01, and 6.18 expression quantity, respectively) in HaCaT cells. On the other hand, genistein has a moderate activity on SIRT1 and SIRT3 with 1.43 and 2.04 expression levels. For the antioxidant activity, the EAS and the pure compounds exhibited stronger antioxidant activity than BHT. While DEF and genistein have a moderate antioxidant activity when compared with BHT. CONCLUSIONS: In this study, the expression levels of SIRT1 and SIRT3 in HaCaT cells increase in the presence of extracts of R. monosperma (L.) Boiss. and the pure compounds.
Ultra-high-performance liquid chromatography - high-resolution mass spectrometry profiling and hepatoprotective activity of purified saponin and flavonoid fractions from the aerial parts of wild spinach (Chenopodium bonus-henricus L.).[Pubmed:33146631]
Z Naturforsch C J Biosci. 2020 Nov 4;76(7-8):261-271.
An ultra-high-performance liquid chromatography - high-resolution mass spectrometry profiling method was used for a comprehensive study of flavonoid and saponin-rich fractions from the aerial parts of wild spinach (Chenopodium bonus-henricus L.). Thirty-six compounds, respectively, 22 saponins of eight sapogenins (phytolaccagenin, bayogenin, medicagenic acid, 2beta-hydroxygypsogenin, 2beta-hydroxyoleanoic acid, 2-hydroxy-30-nor-gypsogenin, 2-hydroxyakebonic acid, and akebonic acid) together with 12 flavonoid glycosides of 6-Methoxykaempferol, isorhamnetin, patuletin, spinacetin as well as two ecdysteroids (20-hydroxyecdysone and polypodine B) were detected. The occurrence of sapogenins 2-hydroxy-30-nor-gypsogenin, 2-hydroxyakebonic acid, and akebonic acid in the Chenopodium genus is reported here for the first time. The flavonoid and saponin-rich fractions showed in vitro hepatoprotective and antioxidant activity comparable to those of flavonoid complex silymarin (60 mug/mL) in a model of metabolic bioactivation, induced by CCl4. All tested fractions, compared to silymarin, significantly reduced the cellular damage caused by CCl4 in rat hepatocytes, preserved cell viability and GSH level, decreased LDH leakage, and reduced lipid damage. The results showed that saponin-rich fractions F3A and F3B possessed better hepatoprotective activity than flavonoid-rich fractions (F2A and F2B). The most active was fraction F3B and this is probably due to the synergism between the saponins and some acylated flavonol glycosides found there.
Neuroprotective, anti-alpha-glucosidase and prolipase active flavonoids from Good King Henry (Chenopodium bonus-henricus L.).[Pubmed:32597284]
Nat Prod Res. 2020 Jun 27:1-5.
Nine glycosides of patuletin, 6-Methoxykaempferol and spinacetin from Good King Henry (Chenopodium bonus-henricus L.) were investigated for neuroprotective, anti-alpha-glucosidase and lipase activities. All tested flavonoids (100 microM) showed statistically significant neuroprotective activities on isolated rat brain synaptosomes using 6-hydroxydopamine in vitro model. They preserved synaptosome viability as well as the reduced glutathione level. 6-Methoxykaempferol glycoside 9 possessed the most prominent neuroprotective and antioxidant effects, within the same range as silibinin (100 microM). Anti-alpha-glucosidase and lipase activities of the tested compounds were established by measuring the levels of the released 4-nitrophenol using LC-MS here for the first time. Patuletin glycosides 2 and 7 possessed similar activity to acarbose with IC50 210, 249 and 206 microM, respectively. All flavonoids exhibited prolipase activity and could be used in the treatment of cachexia. The most active were flavonoids, which contain esterified ferulic acid.
UHPLC-HRMS based flavonoid profiling of the aerial parts of Chenopodium foliosum Asch. (Amaranthaceae).[Pubmed:31711317]
Nat Prod Res. 2021 Oct;35(19):3336-3340.
Chenopodium foliosum Asch. has been recognised by Bulgarian legislation as a medicinal plant. The decoction of its aerial parts has been used for treatment of cancer, as an immunostimulant and antioxidant drug. An UHPLC-HRMS profiling method was used for a comprehensive study of flavonoid composition of C. foliosum. Fourty flavonoid glycosides with nine aglycones (patuletin, gomphrenol, spinacetin, 6-Methoxykaempferol, kaempferol, quercetin, isorhamnetin, 3,5,3',4'-tetrahydroxy-6,7-methylenedioxyflavone and 3,5,4'-trihydroxy-3'-methoxy-6,7-methylenedioxyflavone) were detected. Kaempferol, quercetin and isorhamnetin glycosides were identified as minor components. A pseudo MS(3) experiment aided at discriminating 6-Methoxykaempferol and isorhamnetin glycosides. Flavonoid composition dominated by di-, triglycosides and acylated flavonoids. Acid hydrolysis and GS-MS analysis confirmed the presence of D-glucose, D-apiose and L-rhamnose. Ten flavonoids were reported here for the first time.
6-Methoxyflavonols from the aerial parts of Tetragonia tetragonoides (Pall.) Kuntze and their anti-inflammatory activity.[Pubmed:31003077]
Bioorg Chem. 2019 Jul;88:102922.
Dried aerial parts of Tetragonia tetragonoides were extracted with 70% EtOH, and the evaporated residue was successively separated into EtOAc, n-BuOH, and H2O fractions. As a result of repeated SiO2, ODS, and Sephadex LH-20 column chromatography, four new 6-methoxyflavonol glycosides (2-4, 8) along with four known ones (1, 5-7) were isolated. Several spectroscopic data led to determination of chemical structures for four new 6-methoxyflavonol glycosides (2-4, 8) and four known ones, 6-Methoxykaempferol 3-O-beta-d-glucopyranosyl-(1-->2)-beta-d-glucopyranosyl-7-O-(6''''-(E)-caffeoyl)- beta-d-glucopyranoside (1), 6-methoxyquercetin (5), 6-Methoxykaempferol (6), and 6-Methoxykaempferol 7-O-beta-d-glucopyranoside (7). Methoxyflavonol glycosides 2-8 also have never been reported from T. tetragonoides in this study. 6-Methoxyflavonols 5 and 6 showed high radical scavenging potential in DPPH and ABTS test. Also, all compounds showed significant anti-inflammatory activities such as reduction of NO and PGE2 formation and suppression of TNF-alpha, IL-6, IL-1beta, iNOS, and COX-2 expression in LPS-stimulated RAW 264.7 macrophages. In general, the aglycones exhibited higher activity than the glycosides. In addition, quantitative analysis of 6-methoxyflavonols in the T. tetragonoides aerial parts extract was conducted through HPLC.
Tetragonia tetragonoides (Pall.) Kuntze (New Zealand Spinach) Prevents Obesity and Hyperuricemia in High-Fat Diet-Induced Obese Mice.[Pubmed:30110943]
Nutrients. 2018 Aug 14;10(8). pii: nu10081087.
Tetragonia tetragonoides (Pall.) Kuntze, called New Zealand spinach (NZS), is an edible plant used in salad in Western countries and has been used to treat gastrointestinal diseases in traditional medicine. We examined the anti-obesity and anti-hyperuricemic effects of NZS and the underlying mechanisms in high-fat diet (HFD)-induced obese mice. Mice were fed a normal-fat diet (NFD); high-fat diet (HFD); HFD with 75, 150, or 300 mg/kg NZS extract; or 245 mg/kg Garcinia cambogia (GC) extract. NZS decreased body weight gain, total white adipose tissue (WAT), liver weight, and size of adipocytes and improved hepatic and plasma lipid profiles. With NZS, the plasma levels of the leptin and uric acid were significantly decreased while the levels of the adiponectin were increased. Furthermore, NZS decreased the expression levels of adipogenesis-related genes and xanthine oxidoreductase (XOR), which is involved in uric acid production, while increasing that of proteins associated with fatty acid oxidation. UPLC analysis revealed that NZS contained 6-Methoxykaempferol-3-O-beta-d-glucosyl(1'''-->2'')-beta-d-glucopyranoside, 6-Methoxykaempferol-3-O-beta-d-glucosyl(1'''-->2'')-beta-d-glucopyranosyl-(6''''- caffeoyl)-7-O-beta-d-glucopyranoside, and 6,4'-dimethoxykaempferol-3-O-beta-d-glucosyl(1'''-->2'')-beta-d-glucopyranosyl-(6 ''''-caffeoyl)-7-O-beta-d-glucopyranoside. These results suggest that NZS exerts anti-obesity, anti-hyperlipidemia, and anti-hyperuricemic effects in HFD-induced obese mice, which are partly explained by regulation of lipid-metabolism-related genes and proteins and decreased expression of XOR.
An enhanced targeted identification strategy for the selective identification of flavonoid O-glycosides from Carthamus tinctorius by integrating offline two-dimensional liquid chromatography/linear ion-trap-Orbitrap mass spectrometry, high-resolution diagnostic product ions/neutral loss filtering and liquid chromatography-solid phase extraction-nuclear magnetic resonance.[Pubmed:28256254]
J Chromatogr A. 2017 Mar 31;1491:87-97.
Targeted identification of potentially bioactive molecules from herbal medicines is often stymied by the insufficient chromatographic separation, ubiquitous matrix interference, and pervasive isomerism. An enhanced targeted identification strategy is presented and validated by the selective identification of flavonoid O-glycosides (FOGs) from Carthamus tinctorius. It consists of four steps: (i) enhanced separation and detection by offline two-dimensional liquid chromatography/LTQ-Orbitrap MS (offline 2D-LC/LTQ-Orbitrap MS) using collision-induced dissociation (CID) and high-energy C-trap dissociation (HCD); (ii) improved identification of the major aglycones by acid hydrolysis and LC-SPE-NMR; (iii) simplified spectral elucidation by high-resolution diagnostic product ions/neutral loss filtering; and (iv) more convincing structural identification by matching an in-house library. An offline 2D-LC system configuring an Acchrom XAmide column and a BEH Shield RP-18 UPLC((R)) column enabled much better separation of the easily co-eluting components. Combined use of CID and HCD could produce complementary fragmentation information. The intensity ratios of the aglycone ion species ([Y0-H](-)/Y0(-) and [Y0-2H](-)/Y0(-)) in the HCD-MS(2) spectra were found diagnostic for discriminating the aglycone subtypes and characterizing the glycosylation patterns. Five aglycone structures (kaempferol, 6-hydroxykaempferol, 6-Methoxykaempferol, carthamidin, and isocarthamidin) were identified based on the (1)H-NMR data recorded by LC-SPE-NMR. Of the 107 characterized flavonoids, 80 FOGs were first reported from C. tinctorius. Unknown aglycones, pentose, and novel acyl substituents were discovered. A new compound thereof was isolated and fully identified, which could partially validate the MS-oriented identification. This integral strategy can improve the potency, efficiency, and accuracy in the detection of new compounds from medicinal herbs and other natural sources.
Chenopodium bonus-henricus L. - A source of hepatoprotective flavonoids.[Pubmed:28229939]
Fitoterapia. 2017 Apr;118:13-20.
Three new flavonoid glycosides (7-9) named patuletin-3-O-(5'''-capital O, Cyrillic-capital IE, Cyrillic-feruloyl)-beta-d-apiofuranosyl(1-->2)[beta-d-glucopyranosyl (1-->6)]-beta-d-glucopyranoside (7), spinacetin-3-O-(5'''-capital O, Cyrillic-capital IE, Cyrillic-feruloyl)-beta-d-apiofuranosyl (1-->2)[beta-d-glucopyranosyl(1-->6)]-beta-d-glucopyranoside (8) and 6-Methoxykaempferol-3-O-(5'''-capital O, Cyrillic-capital IE, Cyrillic-feruloyl)-beta-d-apiofuranosyl(1-->2)[beta-d-glucopyranosyl (1-->6)]-beta-d-glucopyranoside (9) together with six known flavonoid glycosides of patuletin, spinacetin and 6-Methoxykaempferol (1-6) were isolated from the aerial parts of C. bonus-henricus and identified with spectroscopic methods (1D and 2D NMR, UV, IR, HRESIMS). The MeOH extract exerts hepatoprotective and antioxidant activities comparable to those of flavonoid complex silymarin in in vitro (60mug/mL) and in vivo (100mg/kg/daily for 7days) models of hepatotoxicity, induced by CCl4. Flavonoids (1-9) (100muM), compared to silybin, significantly reduced the cellular damage caused by CCl4 in rat hepatocytes, preserved cell viability and GSH level, decreased LDH leakage and reduced lipid damage. High concentrations of compounds (1-9) showed marginal or no cytotoxicity on HepG2 cell line. The experiment data suggest that the glycosides of 6-Methoxykaempferol, spinacetin and patuletin are a promising and safe class of hepatoprotective agents.
6-Methoxyflavonol Glycosides with In Vitro Hepatoprotective Activity from Chenopodium bonus-henricus Roots.[Pubmed:26434121]
Nat Prod Commun. 2015 Aug;10(8):1377-80.
One new, namely 6-Methoxykaempferol 3-O-[beta-apiofuranosyl(l-->2)]-f-glucopyranosyl(l->6)-fl-glucopyranoside (2), and two known flavonoid glycosides, spinacetin 3-O-[beta-apiofuranosyl(1-->2)]-beta-glucopyranosyl(1-->6)-beta-glucopyranoside (1) and spinacetin 3-O-gentiobioside (3), were isolated from the roots of Chenopodium bonus-henricus L. Their structures were determined by means of spectroscopic methods (ID, 2D NMR, UV, IR) and HR-ESI-MS. Radical scavenging and anti-oxidant activities of 1 and 3 were established using DPPH and ABTS free radicals, FRAP assay and inhibition of lipid peroxidation (LP) in a linoleic acid system by the ferric thiocyanate method. Compound 3 was found to possess stronger DPPH and ABTS radical scavenging activity (IC50 0.44 +/- 0.008 mM and 0.089 +/- 0.002 mM, respectively) compared with 1 (IC50 1.22 +/- 0.0 10 mM and 0.11 +/- 0.004 mM, respectively). Both flavonoids inhibited the lipid peroxidation of linoleic acid significantly. Additionally, 1 and 3 significantly reduced the cellular damage caused by the hepatotoxic agent CCI4 in rat hepatocytes and preserved cell viability and GSH level, decreased LDH leakage and reduced lipid damage. Effects were similar to those of the positive control silymarin. Control of self-toxic effects made in a MTT based assay using HepG2 cells revealed statistically significant cytotoxic effects only in very high concentrations (exceeding mM) and an incubation time of 72 h, making flavonoid glycosides with a 6-Methoxykaempferol skeleton a promising and safe class of hepatoprotective compounds.
Topical analgesic, anti-inflammatory and antioxidant properties of Oxybaphus nyctagineus: phytochemical characterization of active fractions.[Pubmed:24945398]
J Ethnopharmacol. 2014 Aug 8;155(1):776-84.
ETHNOPHARMACOLOGICAL RELEVANCE: Oxybaphus nyctagineus (Michx.) Sweet has traditionally been used by several Native American tribes predominantly as a topical anti-inflammatory and analgesic agent. AIM OF THE STUDY: To evaluate the antioxidant, analgesic and anti-inflammatory activity of the extracts prepared from the aerial parts of Oxybaphus nyctagineus and to characterize the major chemical constituents of the bioactive extracts. MATERIALS AND METHODS: Crude polar and apolar extracts (PCE and ACE) of the herb of Oxybaphus nyctagineus were prepared and tested in the models of the CFA-induced hyperalgesia in rat knee and carrageenan-induced paw edema in rat. To identify the active compounds, subfractions were prepared by column chromatography and subjected in vitro assays, such as antioxidant assays (DPPH, peroxynitrite (ONOO-) scavenging), and the LPS-induced IL-1beta release test in human monocytes. Preparative HPLC was employed for the isolation of active substances, while phytochemical analysis was performed by mean of LC-MS/MS and NMR. RESULTS: The topically administered PCE and ACE of Oxybaphus nyctagineus demonstrated a significant analgesic and anti-inflammatory effect in the inflammation animal models. The subfraction A4 of ACE and the subfraction P5 of PCE considerably inhibited the LPS-induced IL-1beta release in human monocytes, while the strongest activity was localized in the subfraction P5 in the antioxidant assays. The HPLC-MS/MS and NMR analysis revealed that 6-methoxyflavonol diglycosides, namely patuletin-3-O-robinobioside (1), 6-Methoxykaempferol-3-O-robinobioside (2), spinacetin-3-O-robinobioside (3), and hydroxy-polyenoic fatty acids, namely corchorifatty acid B (4), 9-hydroxy-10E,12Z,15Z-octadecatrienoic acid (9-HOT acid) (5), and 9-hydroxy-10E,12Z-octadecadienoic acid (9-HOD acid) (6) were present in PCE, and in ACE as major compounds. CONCLUSION: The results of this study established a pharmacological evidence for the traditional use of Oxybaphus nyctagineus as an anti-inflammatory agent used topically, and provided data on its phytochemical composition for the first time.
Determination of major components and fingerprint analysis of Flaveria bidentis (L.) Kuntze.[Pubmed:23515193]
J Chromatogr Sci. 2014 Mar;52(3):252-7.
A sensitive high-performance liquid chromatography method coupled with photodiode array detection was developed for the simultaneous determination of six major constituents in Flaveria bidentis (L.) Kuntze: hyperoside, patuletin-3-O-glucoside, isorhamnetin 3-sulfate, astragalin, 6-Methoxykaempferol-3-O-galactoside and alpha-terthienyl. The chemical fingerprint of Flaveria bidentis (L.) Kuntze leaves was established using raw materials of 12 batches in China. The chromatographic separations were obtained by using an Eclipse XDB-C18 reserved-phase column (150 x 4.6 mm i.d., 5 microm) using gradient elution with water (0.0125% trifluoroacetic acid, v/v) and acetonitrile at a flow rate of 1.0 mL/min, an operating temperature of 30 degrees C and a detection wavelength of 360 nm. The new method was validated by linearity, limits of detection and quantification, precision, reproducibility, stability and recovery, and was also successfully applied to the simultaneous determination of components in Flaveria bidentis (L.) Kuntze. The results indicate that this multi-component determination method in combination with chromatographic fingerprint analysis is suitable for the quantitative analysis and identification of Flaveria bidentis (L.) Kuntze.
Bioactivity-guided isolation of antiproliferative compounds from Centaurea jacea L.[Pubmed:22537643]
Fitoterapia. 2012 Jul;83(5):921-5.
Bioassay-guided fractionation of the chloroform extract of Centaurea jacea L. afforded the isolation of cirsiliol, apigenin, hispidulin, eupatorin, isokaempferide, axillarin, centaureidin, 6-Methoxykaempferol 3-methyl ether, trachelogenin, cnicin, 4'-acetylcnicin and three aliphatic glucose diesters, including the new natural product 1beta-isobutanoyl-2-angeloyl-glucose. The structures of the compounds were established on the basis of spectroscopic analyses (UV, MS and NMR). All compounds were isolated for the first time from this species. The compounds were evaluated for their tumour cell growth inhibitory activities on HeLa, MCF-7 and A431 cells. Different types of secondary metabolites (flavonoids, sesquiterpenes) were found to be responsible for the antitumour effects of the extracts; the highest activity was exerted by centaureidin, in addition to moderately active compounds (cirsiliol, isokaempferide, apigenin, hispidulin, cnicin and 4'-acetylcnicin).
Flavonoids from Algerian endemic Centaurea microcarpa and their chemotaxonomical significance.[Pubmed:22224272]
Nat Prod Commun. 2011 Nov;6(11):1603-4.
Six flavonoids, namely 6-Methoxykaempferol (1), 6-Methoxykaempferol 7-O-glucoside (2), kaempferol 7-O-glucoside (3), 6-methoxyluteolin (4), patuletin 7-O-glucoside (5), and hispidulin 7-O-glucoside (6), were isolated from a n-butanolic fraction of Centaurea microcarpa Coss et Dur. flowers. This work describes for the first time the phytochemical composition of this endemic Algerian plant.
beta(2)-Adrenergic activity of 6-methoxykaempferol-3-O-glucoside on rat uterus: in vitro and in silico studies.[Pubmed:21663739]
Eur J Pharmacol. 2011 Sep 30;667(1-3):348-54.
6-Methoxykaempferol-3-O-glucoside (6-MKG) was isolated from a Sudanese herb (El-hazha). The pharmacological effects of 6-MKG were tested on isolated non-pregnant or late-pregnant rat uteri in vitro, whilst docking studies were carried out modelling of the binding of 6-MKG to the rat beta(2)-adrenoceptor in silico. In vitro studies revealed that 6-MKG was able to relax both the non-pregnant and the late-pregnant uterine contractility with 50% of the E(max) of terbutaline, whilst the EC(50) for 6-MKG was at least half than that of terbutaline. The beta(2)-adrenoceptors antagonist 3-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol(ICI118,551) competitively antagonised the relaxing effect of 6-MKG. Radioligand binding and cAMP studies confirmed the beta(2)-adrenoceptors agonistic property of the compound. In in silico docking studies, 6-MKG bound to rat beta(2)-adrenoceptors with low G(bind) value (-11.53+/-0.06 kcal/mol) and it interacted with four residues of the active site (Asp(113), Asn(312), Cys(191)and Tyr(316)). It is concluded that 6-MKG exerts weak beta(2)-adrenoceptor agonistic activity and can be considered a natural compound with potential therapeutic significance in the field of premature pregnant uterine contractions and asthmatic problems.
Separation of patuletin-3-O-glucoside, astragalin, quercetin, kaempferol and isorhamnetin from Flaveria bidentis (L.) Kuntze by elution-pump-out high-performance counter-current chromatography.[Pubmed:21329934]
J Chromatogr A. 2011 Sep 9;1218(36):6206-11.
Flaveria bidentis (L.) Kuntze is an annual alien weed of Flaveria Juss. (Asteraceae) in China. Bioactive compounds, mainly flavonol glycosides and flavones from F. bidentis (L.) Kuntze, have been studied in order to utilize this invasive weed, Analytical high-performance counter-current chromatography (HPCCC) was successfully used to separate patuletin-3-O-glucoside, a mixture of hyperoside (quercetin-3-O-galactoside) and 6-Methoxykaempferol-3-O-galactoside, astragalin, quercetin, kaempferol and isorhamnetin using two runs with different solvent system. Ethyl acetate-methanol-water (10:1:10, v/v) was selected by analytical HPCCC as the optimum phase system for the separation of patuletin-3-O-glucoside, a mixture of hyperoside and 6-Methoxykaempferol-3-O-galactoside, and astragalin. A Dichloromethane-methanol-water (5:3:2, v/v) was used for the separation of quercetin, kaempferol and isorhamnetin. The separation was then scaled up: the crude extract (ca 1.5 g) was separated by preparative HPCCC, yielding 12 mg of patuletin-3-O-glucoside at a purity of 98.3%, yielding 9 mg of a mixture of hyperoside and 6-Methoxykaempferol-3-O-galactoside constituting over 98% of the fraction, and 16 mg of astragalin (kaempferol-3-O-glucoside) at a purity of over 99%. The pump-out peaks are isorhanetin (98% purity), kaemferol (93% purity) and quercitin (99% purity). The chemical structure of patuletin-3-O-glucoside and astragalin were confirmed by MS and (1)H, (1)(3)C NMR.


