VolkensiflavoneCAS# 27542-37-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 27542-37-6 | SDF | Download SDF |
| PubChem ID | 23844069 | Appearance | Yellow powder |
| Formula | C30H20O10 | M.Wt | 540.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
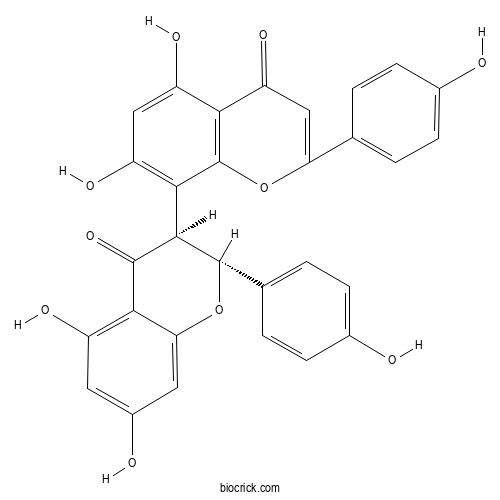
| Chemical Name | 8-[(2R,3S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-2,3-dihydrochromen-3-yl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one | ||
| SMILES | C1=CC(=CC=C1C2C(C(=O)C3=C(C=C(C=C3O2)O)O)C4=C(C=C(C5=C4OC(=CC5=O)C6=CC=C(C=C6)O)O)O)O | ||
| Standard InChIKey | YOGANETYFUQWIM-PXJZQJOASA-N | ||
| Standard InChI | InChI=1S/C30H20O10/c31-15-5-1-13(2-6-15)22-12-21(37)24-19(35)11-20(36)26(30(24)39-22)27-28(38)25-18(34)9-17(33)10-23(25)40-29(27)14-3-7-16(32)8-4-14/h1-12,27,29,31-36H/t27-,29+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Volkensiflavone Dilution Calculator

Volkensiflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8501 mL | 9.2507 mL | 18.5014 mL | 37.0028 mL | 46.2535 mL |
| 5 mM | 0.37 mL | 1.8501 mL | 3.7003 mL | 7.4006 mL | 9.2507 mL |
| 10 mM | 0.185 mL | 0.9251 mL | 1.8501 mL | 3.7003 mL | 4.6253 mL |
| 50 mM | 0.037 mL | 0.185 mL | 0.37 mL | 0.7401 mL | 0.9251 mL |
| 100 mM | 0.0185 mL | 0.0925 mL | 0.185 mL | 0.37 mL | 0.4625 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,8-Dihydroxy-6,7-dimethoxyflavone
Catalog No.:BCN0676
CAS No.:73202-52-5
- Nuchensin
Catalog No.:BCN0675
CAS No.:25782-24-5
- 2'-Hydroxyneophellamuretin
Catalog No.:BCN0674
CAS No.:1396769-20-2
- 6-Methoxykaempferol 3-O-glucoside
Catalog No.:BCN0673
CAS No.:63422-27-5
- 6-Methoxykaempferol
Catalog No.:BCN0672
CAS No.:32520-55-1
- 5,6-Dihydroxy-3,7,4'-trimethoxyflavone
Catalog No.:BCN0671
CAS No.:84019-17-0
- 5,6-Dihydroxy-3,7,3',4'-tetramethoxyflavone
Catalog No.:BCN0670
CAS No.:63296-15-1
- 3,5,8,3',4',5'-Hexamethoxy-6,7-methylenedioxyflavone
Catalog No.:BCN0669
CAS No.:82668-95-9
- 7,4'-Dihydroxy-6,8-diprenylflavanone
Catalog No.:BCN0668
CAS No.:50939-03-2
- Isosativanone
Catalog No.:BCN0667
CAS No.:82829-55-8
- Hydnocarpin
Catalog No.:BCN0666
CAS No.:51419-48-8
- Kaempferol 3-O-(3",6"-di-O-E-p-coumaroyl)-β-D-glucopyranoside
Catalog No.:BCN0665
CAS No.:218605-31-3
- Quercitrin 2''-O-arabinoside
Catalog No.:BCN0678
CAS No.:104683-55-8
- Mikanin
Catalog No.:BCN0679
CAS No.:4324-53-2
- 6'-Prenylisorhamnetin
Catalog No.:BCN0680
CAS No.:1859979-02-4
- 2'-Prenylisorhamnetin
Catalog No.:BCN0681
CAS No.:1932668-04-6
- Orientin 2''-O-rhamnoside
Catalog No.:BCN0682
CAS No.:81398-30-3
- Naringenin 7-O-β-D-glucuronide methyl ester
Catalog No.:BCN0683
CAS No.:1985597-72-5
- Eriodictyol 7-O-β-D-glucuronide ethyl ester
Catalog No.:BCN0684
CAS No.:847025-48-3
- 8-(1,1-Dimethyl-2-propenyl)-3'-methoxykaempferol
Catalog No.:BCN0685
CAS No.:1859979-00-2
- Eriodictyol 7-O-methylglucuronide
Catalog No.:BCN0686
CAS No.:133360-42-6
- 8-(1,1-Dimethyl-2-propenyl)kaempferol
Catalog No.:BCN0687
CAS No.:142646-43-3
- 3,5,7-Trihydroxy-8-methoxyflavone
Catalog No.:BCN0688
CAS No.:5928-42-7
- 2''-O-E-p-Coumaroylafzelin
Catalog No.:BCN0689
CAS No.:151455-10-6
Biflavones from Platonia insignis Mart. Flowers Promote In Vitro Antileishmanial and Immunomodulatory Effects against Internalized Amastigote Forms of Leishmania amazonensis.[Pubmed:34578198]
Pathogens. 2021 Sep 10;10(9). pii: pathogens10091166.
Leishmaniasis is an infectious disease that affects millions of people worldwide, making the search essential for more accessible treatments. The species Platonia insignis Mart. (Clusiaceae) has been extensively studied and has gained prominence for its pharmacological potential. The objective of this work was to evaluate the antileishmania activity, cytotoxic effect and activation patterns of macrophages of hydroalcoholic extract (EHPi), ethyl acetate fractions (FAcOEt) and morelloflavone/Volkensiflavone mixture (MB) from P. insignis flowers. EHPi, FAcOEt and MB demonstrated concentration-dependent antileishmania activity, with inhibition of parasite growth in all analyzed concentrations. EHPi exhibited maximum effect at 800 mug/mL, while FAcOEt and MB reduced the growth of the parasite by 94.62% at 800 mug/mL. EHPi, FAcOEt and MB showed low cytotoxic effects for macrophages at 81.78, 159.67 and 134.28 mug/mL, respectively. EHPi (11.25 microg/mL), FAcOEt (11.25 and 22.5 microg/mL) and MB (22.5 microg/mL) characterized the increase in lysosomal activity, suggesting a possible modulating effect. These findings open for the application of flowers from a P. insignis flowers and biflavones mixture thereof in the promising treatment of leishmaniasis.
Modulation of the Drug Resistance by Platonia insignis Mart. Extract, Ethyl Acetate Fraction and Morelloflavone/Volkensiflavone (Biflavonoids) in Staphylococcus aureus Strains Overexpressing Efflux Pump Genes.[Pubmed:32445452]
Curr Drug Metab. 2021;22(2):114-122.
BACKGROUND: Microbial resistance to antibiotics is a global public health problem, which requires urgent attention. Platonia insignis is a native species from the eastern Brazilian Amazon, used in the treatment of burns and wounds. OBJECTIVES: To evaluate the antimicrobial activity of the hydroalcoholic extract of P. insignis (PIHA), the ethyl acetate fraction (PIAE), and its subfraction containing a mixture of biflavonoids (BF). Moreover, the effect of these natural products on the antibiotic activity against S. aureus strains overexpressing efflux pump genes was also evaluated. METHODS: Minimal inhibitory concentrations were determined against different species of microorganisms. To evaluate the modulatory effect on the Norfloxacin-resistance, the MIC of this antibiotic was determined in the absence and presence of the natural products at subinhibitory concentrations. Inhibition of the EtBr efflux assays were conducted in the absence or presence of natural products. RESULTS: PIHA showed a microbicidal effect against S. aureus and C. albicans, while PIAE was bacteriostatic for S. aureus. PIAE and BF at subinhibitory concentrations were able to reduce the MIC of Norfloxacin acting as modulating agents. BF was able to inhibit the efflux of EtBr efflux in S. aureus strains overexpressing specific efflux pump genes. CONCLUSION: P. inignisis, a source of efflux pump inhibitors, including Volkensiflavone and morelloflavone, which were able to potentiate the Norfloxacin activity by NorA inhibition, being also able to inhibit QacA/B, TetK and MsrA. Volkensiflavone and morelloflavone could be used as an adjuvant in the antibiotic therapy of multidrug resistant S. aureus strains overexpressing efflux pumps.
Effects of biflavonoids from Garcinia madruno on a triple transgenic mouse model of Alzheimer's disease.[Pubmed:29229355]
Pharmacol Res. 2018 Mar;129:128-138.
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that is pathologically characterized by the deposition of beta-amyloid (betaA) peptides in senile plaques and neurofibrillary tangles in the brain. Flavonoids have recently been used to prevent and treat a variety of neurodegenerative diseases, but little is known about bioflavonoids. In this study, we evaluate whether a biflavonoid fraction (BF) exerts neuroprotective effects on an aged triple transgenic mouse mode of AD (3xTg-AD). Then, 21-24-month-old 3xTg AD mice were i.p. injected with 25mg/kg of a BF from Garcinia madruno composed of morelloflavone (65%), Volkensiflavone (12%), GB 2a (11%), fukugiside (6%) and amentoflavone (0.4%) every 48h for 3 months. The BF treatment reduced betaA deposition in different regions of the brain (the hippocampus, entorhinal cortex and amygdala), reduced betaA1-40 and betaA1-42 levels, BACE1-mediated cleavage of APP (CTFbeta), tau pathology, astrogliosis and microgliosis in the brains of aged 3xTg-AD mice. Although the BF treatment weakly improved learning, animals treated with BF spent more time in the open arms of the elevated plus maze test and displayed greater risk assessment behavior than the control groups. In summary, the BF reverses histopathological hallmarks and reduces emotional disorders in the 3xTg-AD mouse model, suggesting that the biflavonoids from G. madruno represent a potential natural therapeutic option for AD if its bioavailability is improved.
Using Ultra-Performance Liquid Chromatography Quadrupole Time of Flight Mass Spectrometry-Based Chemometrics for the Identification of Anti-angiogenic Biflavonoids from Edible Garcinia Species.[Pubmed:28926234]
J Agric Food Chem. 2017 Sep 27;65(38):8348-8355.
Garcinia xanthochymus fruits are edible and also used in traditional medicine. Our previous work showed that the isolated natural products from G. xanthochymus fruits have displayed antioxidant activity and cytotoxicity in the colon cancer cells. In this study, we developed a strategy to correlate a zebrafish angiogenesis assay with ultra-performance liquid chromatography quadrupole time of flight mass spectrometry-based chemometric analysis to identify potential anti-angiogenic activity compounds from G. xanthochymus fruits. Primary bioactivity results showed that the methanolic extracts from aril and pericarp but not from seed have significant inhibitory effects on the growth of subintestinal vessels (SIVs) in zebrafish embryos. A total of 13 markers, including benzophenones and biflavonoids, were predicted by untargeted principal component analysis and orthogonal partial least squares discriminate analysis, which were tentatively identified as priority markers for the bioactivity related in aril and pericarp. Amentoflavone, a biflavonoid, has been found to significantly inhibit the growth of SIVs at 10 and 20 muM and downregulate the expressions of Angpt2 and Tie2 genes of zebrafish embryos. Furthermore, seven biflavonoids, Volkensiflavone, fukugetin, fukugeside, GB 1a, GB 1a glucoside, GB 2a, and GB 2a glucoside, isolated from Garcinia species were evaluated for their structure-activity relationship using the zebrafish model. Only fukugetin, which was previously shown to be anticancer, was active in inhibiting the SIV growth. In this report, both amentoflavone and fukugetin, for the first time, displayed anti-angiogenic effects on zebrafish, thus demonstrating an effective and rapid strategy to identify natural products for anti-angiogenesis activity.
Natural Biflavonoids Modulate Macrophage-Oxidized LDL Interaction In Vitro and Promote Atheroprotection In Vivo.[Pubmed:28824646]
Front Immunol. 2017 Aug 4;8:923.
The accumulation of oxidized ApoB-100-containing lipoproteins in the vascular intima and its subsequent recognition by macrophages results in foam cell formation and inflammation, key events during atherosclerosis development. Agents targeting this process are considered potentially atheroprotective. Since natural biflavonoids exert antioxidant and anti-inflammatory effects, we evaluated the atheroprotective effect of biflavonoids obtained from the tropical fruit tree Garcinia madruno. To this end, the pure biflavonoid aglycones morelloflavone (Mo) and Volkensiflavone (Vo), as well as the morelloflavone's glycoside fukugiside (Fu) were tested in vitro in primary macrophages, whereas a biflavonoid fraction with defined composition (85% Mo, 10% Vo, and 5% Amentoflavone) was tested in vitro and in vivo. All biflavonoid preparations were potent reactive oxygen species (ROS) scavengers in the oxygen radical absorbance capacity assay, and most importantly, protected low-density lipoprotein particle from both lipid and protein oxidation. In biflavonoid-treated macrophages, the surface expression of the oxidized LDL (oxLDL) receptor CD36 was significantly lower than in vehicle-treated macrophages. Uptake of fluorescently labeled oxLDL and cholesterol accumulation were also attenuated in biflavonoid-treated macrophages and followed a pattern that paralleled that of CD36 surface expression. Fu and Vo inhibited oxLDL-induced ROS production and interleukin (IL)-6 secretion, respectively, whereas all aglycones, but not the glucoside Fu, inhibited the secretion of one or more of the cytokines IL-1beta, IL-12p70, and monocyte chemotactic protein-1 (MCP-1) in lipopolysaccharide (LPS)-stimulated macrophages. Interestingly, in macrophages primed with low-dose LPS and stimulated with cholesterol crystals, IL-1beta secretion was significantly and comparably inhibited by all biflavonoid preparations. Intraperitoneal administration of the defined biflavonoid fraction into ApoE(-/-) mice was atheroprotective, as evidenced by the reduction of the atheromatous lesion size and the density of T cells and macrophages infiltrating the aortic root; moreover, this treatment also lowered the circulating levels of cholesterol and the lipid peroxidation product malondialdehyde. These results reveal the potent atheroprotective effects exerted by biflavonoids on key events of the oxLDL-macrophage interphase: (i) atheroligand formation, (ii) atheroreceptor expression, (iii) foam cell transformation, and (iv) prooxidant/proinflammatory macrophage response. Furthermore, our results also evidence the antioxidant, anti-inflammatory, hypolipemiant, and atheroprotective effects of Garcinia madruno's biflavonoids in vivo.
Polyphenols from Allanblackia floribunda seeds: Identification, quantification and antioxidant activity.[Pubmed:28041556]
Food Chem. 2017 May 1;222:35-42.
Oil rich seeds of Allanblackia floribunda, a tree from tropical Africa, have traditionally been used in food preparation. Furthermore, the therapeutic properties of various parts of this tree have long been exploited in traditional medicine. As both food and pharmaceutical industries show growing interest in tropical tree crops, this study aimed to investigate whether A. floribunda seeds could also be used as a source of potentially bioactive compounds. The polyphenol profile revealed six predominant compounds which were identified by HPLC-PDA-ESI/MS(n) as the biflavonoids morelloflavone, Gb-2a and Volkensiflavone and their respective glucosides. A range of less abundant flavones, flavonols and flavan-3-ols was also detected. All six major compounds showed antioxidant activity, with the activity of morelloflavone, its glucoside and Gb-2a-glucoside comparable with that of ascorbic acid. The main compounds accounted for approximately 10% of dry weight, making the seeds used for oil production a rich source of biflavonoids as a by-product.
Allanblackia floribunda Oliv.: An aphrodisiac plant with vasorelaxant properties.[Pubmed:27647010]
J Ethnopharmacol. 2016 Nov 4;192:480-485.
ETHNOPHARMACOLOGICAL RELEVANCE: Allanblackia floribunda Oliv. is one of the most commonly used medicinal plant in Cameroon. The stem bark of the plant is traditionally used for its aphrodisiac and antihypertensive properties. AIM OF THE STUDY: To validate the traditional uses of Allanblackia floribunda stem bark ethanol extract through the evaluation of their aphrodisiac and vasorelaxant properties. MATERIALS AND METHODS: The extract's ability to increase sexual desire and the frequencies of erection (mount), intromission and prolonged latency of ejaculation were studied on adult male rats. The vasodilator effect was investigated using isolated rat aorta rings. Tests were conducted using fractions obtained by reverse phase column-chromatography (CC), after the acquisition of the HPLC fingerprint of the ethanol extract, resulted the most active in previous studies. RESULTS: The CC allowed the isolation of five fractions whose aphrodisiac and vasodilator activities were tested and compared with those of the whole extract. Four compounds were identified and characterized, three of them, Fukugiside, Morelloflavone and Volkensiflavone, are secondary metabolites known to be in Allanblackia floribunda; the fourth, Spicataside, is a biflavonoid glycoside known to be present in the genus Garcinia but never found neither in Allanblackia floribunda nor in Allanblackia genus. The crude ethanolic extract (CEE) induced a relaxation on aorta rings with EC50=11+/-2mug/mL and Morelloflavone displayed a similar activity with EC50=42+/-6mug/mL; for all the other compounds only the vasodilation % at the maximum concentration assessable (90mug/mL) was determined: 30+/-8 (Fukugiside), 24+/-6 (Spicataside), 33+/-4 (Morelloflavone+Volkensiflavone), 47+/-1 (Volkensiflavone). Regarding the activity on male sexual behaviour, only CEE and Fukugiside showed activity in the 9 parameters evaluated. CONCLUSIONS: These results may support the traditional uses of Allanblackia floribunda as aphrodisiac plant with antihypertensive properties suggesting the phytocomplex as responsible for the claimed activity.
[Chemical constituents from leaves of Garcinia xanthochymus].[Pubmed:28901107]
Zhongguo Zhong Yao Za Zhi. 2016 Jun;41(11):2098-2104.
The constituents were isolated and purified by the silica gel and semi-preparative HPLC, and their structures were elucidated by NMR spectral and MS data. Fifteen compounds were isolated from the ethyl acetate fraction of 95% ethanol extract from the leaves of Garcinia xanthochymus, and identified as 5, 7, 4'-trihydroxy-6-(3-hydroxy-3-methylbutyl)-flavone(1), 1,5-dihydroxy-3-methoxyxanthone(2), 1, 3-dimethoxy-5-hydroxy xanthone(3), kaempferol(4),(2S,3S)-trans-dihydrokaempferol(5), 3, 24, 25-trihydroxytirucall-7-ene(6), 4-hydroxycinnamic acid(7), isovanillic acid(8),(Z)-2-(2,4-dihydroxy-2, 6, 6-trimethylcyclohexylidene)acetic acid(9), Volkensiflavone(10), morelloflavone(11), 3, 8''-biapigenin(12), bilobetin(13), fukugiside(14), GB2a glucoside(15). Compound 1 is a new compound, compounds 5, 6, 9 and 13 are isolated from the genus Garcinia for the first time, and compounds 4, 7-8, 10-12, 14 and 15 are firstly found from this plant. alpha-Amylase inhibitory activities of 10 compounds were determined using starch azure as the substrate, and the results show that compound 13 has the inhibitory activities against alpha-amylase, IC(5)(0) values of compound 13 and acarbose are 8.12, 4.32 mumol*L(-)(1) respectively.
Antiplasmodial activity of some phenolic compounds from Cameroonians Allanblackia.[Pubmed:26957972]
Afr Health Sci. 2015 Sep;15(3):835-40.
BACKGROUND: Plasmodium falciparum, one of the causative agents of malaria, has high adaptability through mutation and is resistant to many types of anti-malarial drugs. This study presents an in vitro assessment of the antiplasmodial activity of some phenolic compounds isolated from plants of the genus Allanblackia. METHODS: Tests were performed on well plates filled with a fixed parasitized erythrocytes volume. Compounds to be tested were then added in wells. After incubation, tritiated hypoxanthine is added and the plates were returned to the incubator. After thawing, the nucleic acids are collected. Inhibitory Concentration 50 (IC50) was determined by linear interpolation. RESULTS: From Allanblackia floribunda, have been isolated and characterized 1,7-dihydroxyxanthone 1, macluraxanthone 4, morelloflavone 9, Volkensiflavone 10 and morelloflavone 7-O-glucoside 11; from Allanblackia monticola, alpha-mangosine 2, rubraxanthone 3, allaxanthone C 5, norcowanine 6, tovophiline A 7, allaxanthone B 8 and from Allanblackia gabonensis, 1,7-dihydroxyxanthone 1. Six of them were evaluated for their antimalarial properties. The most active compound, macluraxanthone, presented a very interesting activity, with an IC50 of 0.36 and 0.27 microg/mL with the F32 and FcM29 strains respectively. CONCLUSION: This work confirms that species of Allanblackia genus are medicinally important plants containing many biologically active compounds that can be used effectively as antiplasmodial.
UPLC-ESI-TOF MS-Based Metabolite Profiling of the Antioxidative Food Supplement Garcinia buchananii.[Pubmed:26226176]
J Agric Food Chem. 2015 Aug 19;63(32):7169-79.
Comparative antioxidative analyses of aqueous ethanolic extracts from leaf, root, and stem of Garcinia buchananii revealed high activity of all three organs. To investigate the metabolite composition of the different parts of G. buchananii, an untargeted metabolomics approach using UPLC-ESI-TOF MS with simultaneous acquisition of low- and high-collision energy mass spectra (MS(e)) was performed. Unsupervised statistics (PCA) highlighted clear differences in the metabolomes of the three organs. OPLS-DA revealed (2R,3S,2''R,3''R)-GB-1, (2R,3S)-morelloflavone, and (2R,3S)-Volkensiflavone as the most decisive marker compounds discriminating leaf from root and stem extract. Leaves represent the best source to isolate GB-1, morelloflavone, and Volkensiflavone. Root extract is the best organ to isolate xanthones and stem bark extract the best source to isolate (2R,3S,2''R,3''R)-manniflavanone; the identified polyisoprenylated benzophenones are characteristic compounds for the leaf organ. Morelloflavone, Volkensiflavone, and garcicowin C were isolated for the first time from G. buchananii, identified via MS, NMR, and CD spectroscopy, and showed in H2O2 scavenging, H/L-TEAC, and H/L-ORAC assays moderate to strong in vitro antioxidative activities.
A bioactive cycloartane triterpene from Garcinia hombroniana.[Pubmed:26158779]
Nat Prod Res. 2016 Jun;30(12):1388-97.
The dichloromethane bark extract of Garcinia hombroniana yielded one new cycloartane triterpene; (22Z,24E)-3beta-hydroxycycloart-14,22,24-trien-26-oic acid (1) together with five known compounds: garcihombronane G (2), garcihombronane J (3), 3beta acetoxy-9alpha-hydroxy-17,14-friedolanostan-14,24-dien-26-oic acid (4), (22Z, 24E)-3beta, 9alpha-dihydroxy-17,14-friedolanostan-14,22,24-trien-26-oic acid (5) and 3beta, 23alpha-dihydroxy-17,14-friedolanostan-8,14,24-trien-26-oic acid (6). Their structures were established by the spectral techniques of NMR and ESI-MS. These compounds together with some previously isolated compounds; garcihombronane B (7), garcihombronane D (8) 2,3',4,5'-tetrahydroxy-6-methoxybenzophenone (9), Volkensiflavone (10), 4''-O-methyll-Volkensiflavone (11), Volkensiflavone-7-O-glucopyranoside (12), Volkensiflavone-7-O-rhamnopyranoside (13), Morelloflavone (14), 3''-O-methyl-morelloflavone (15) and morelloflavone-7-O-glucopyranoside (16) were evaluated for cholinesterase enzymes inhibitory activities using acetylcholinesterase and butyrylcholinesterase. In these activities, compounds 1-9 showed good dual inhibition on both the enzymes while compounds 10-16 did not reasonably contribute to both the cholinesterases inhibitory effects.
Complete NMR assignments of bioactive rotameric (3 --> 8) biflavonoids from the bark of Garcinia hombroniana.[Pubmed:24700704]
Magn Reson Chem. 2014 Jul;52(7):345-52.
The genus Garcinia is reported to possess antimicrobial, anti-inflammatory, anticancer, hepatoprotective and anti-HIV activities. Garcinia hombroniana in Malaysia is used to treat itching and as a protective medicine after child birth. This study was aimed to isolate the chemical constituents from the bark of G. hombroniana and explore their possible pharmacological potential. Ethyl acetate extract afforded one new (1) and six (2-7) known 3 --> 8 rotameric biflavonoids. Their structures were elucidated by UV, IR and NMR (1D and 2D) spectroscopy together with electron ionization/ESI mass spectrometric techniques and were identified as (2R, 3S) Volkensiflavone-7-O-rhamnopyranoside (1), Volkensiflavone (2), 4''-O-methyl-Volkensiflavone (3), Volkensiflavone-7-O-glucopyranoside (4), morelloflavone (5), 3''-O-methyl-morelloflavone (6) and morelloflavone-7-O-glucopyranoside (7). The absolute configuration of compound 1 was assigned by circular dichroism spectroscopy as 2R, 3S. The coexistence of conformers of isolated biflavonoids in solution at 25 degrees C in different solvents was confirmed by variable temperature NMR studies. At room temperature (25 degrees C), compounds 1-7 exhibited duplicate NMR signals, while at elevated temperature (90 degrees C), a single set of signals was obtained. Compound 5 showed significant in vitro antioxidant activities against 1,1-diphenyl-2-picrylhydrazyl and 2,2'-azino-bis-3-ethyl benzthiazoline-6-sulfonic acid radicals. The antibacterial studies showed that compounds 5 and 6 are the most active against Staphylococcus aureus, Bacillus subtilis and Escherichia coli. Compounds 3 and 6 also showed moderate antituberculosis activity against H38 Rv. Based on the research findings, G. hombroniana could be concluded as a rich source of flavanone-flavone (3 --> 8) biflavonoids that exhibit rotameric behaviour at room temperature and display significant antioxidant and antibacterial activities.
Biflavonoids, main constituents from Garcinia bakeriana leaves.[Pubmed:24273855]
Nat Prod Commun. 2013 Sep;8(9):1237-40.
The genus Garcinia is a source of a large variety of organic compounds including biflavonoids, acylphloroglucinols and xanthones mainly, but few data are available about the chemical composition of Cuban species. The aim of this investigation was to identify the main constituents of G. bakeriana Urb., a rare Cuban endemic plant. A new biflavonoid, 4'''-O-methyl-I3,II8-biapigenin (1), together with 9 known compounds, namely, the biflavonoids amentoflavone (2), 4'''-O-methylamentoflavone (3), 4'-O-methylcupressuflavone (4), GB-2a (5), Volkensiflavone (6), 6"-(2-hydroxy-3-methyl-3-butenyl)-amentoflavone (7), I3,II8-biapigenin (8), and GB-1a (9), and the xanthone norathyriol (10), were isolated from the leaves of this species. All the structures were elucidated by spectroscopic methods including 1D and 2D NMR experiments, as well as ESIMS analysis. These results showed that the isolated biflavonoids possess a C-C interflavonoid linkage between the apigenin units or its derivatives.
Benzophenones and biflavonoids from Rheedia edulis.[Pubmed:21028890]
J Nat Prod. 2010 Nov 29;73(11):1775-9.
Two new polyisoprenylated benzophenones, 32-hydroxy-ent-guttiferone M (1) and 6-epi-guttiferone J (2), along with seven known compounds, 6-epi-clusianone (3), guttiferone A (4), xanthochymol (5), guttiferone E (6), isoxanthochymol (7), (+)-Volkensiflavone (8), and (+)-morelloflavone (9), were identified from the seeds and rinds of Rheedia edulis. Compounds 1-3 and 5-9 have been isolated and identified from this species for the first time. The structures of the new compounds were elucidated mainly by analysis of their 1D and 2D NMR spectroscopic data, and their absolute configurations were determined by comparison of their experimental optical rotation and electronic circular dichroism measurements with those values predicted by DFT calculations. Compound 1 showed significant antioxidant activity in both DPPH and ABTS free radical scavenging assays, whereas compound 2 was inactive.


