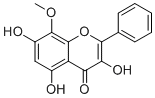
3,5,7-Trihydroxy-8-methoxyflavoneCAS# 5928-42-7 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 5928-42-7 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Yellow powder |
| Formula | C16H12O6 | M.Wt | 300.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 3-Hydroxywogonin; 8-Methoxygalangin | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3,5,7-Trihydroxy-8-methoxyflavone Dilution Calculator

3,5,7-Trihydroxy-8-methoxyflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.33 mL | 16.65 mL | 33.3 mL | 66.6001 mL | 83.2501 mL |
| 5 mM | 0.666 mL | 3.33 mL | 6.66 mL | 13.32 mL | 16.65 mL |
| 10 mM | 0.333 mL | 1.665 mL | 3.33 mL | 6.66 mL | 8.325 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.332 mL | 1.665 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.666 mL | 0.8325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 8-(1,1-Dimethyl-2-propenyl)kaempferol
Catalog No.:BCN0687
CAS No.:142646-43-3
- Eriodictyol 7-O-methylglucuronide
Catalog No.:BCN0686
CAS No.:133360-42-6
- 8-(1,1-Dimethyl-2-propenyl)-3'-methoxykaempferol
Catalog No.:BCN0685
CAS No.:1859979-00-2
- Eriodictyol 7-O-β-D-glucuronide ethyl ester
Catalog No.:BCN0684
CAS No.:847025-48-3
- Naringenin 7-O-β-D-glucuronide methyl ester
Catalog No.:BCN0683
CAS No.:1985597-72-5
- Orientin 2''-O-rhamnoside
Catalog No.:BCN0682
CAS No.:81398-30-3
- 2'-Prenylisorhamnetin
Catalog No.:BCN0681
CAS No.:1932668-04-6
- 6'-Prenylisorhamnetin
Catalog No.:BCN0680
CAS No.:1859979-02-4
- Mikanin
Catalog No.:BCN0679
CAS No.:4324-53-2
- Quercitrin 2''-O-arabinoside
Catalog No.:BCN0678
CAS No.:104683-55-8
- Volkensiflavone
Catalog No.:BCN0677
CAS No.:27542-37-6
- 5,8-Dihydroxy-6,7-dimethoxyflavone
Catalog No.:BCN0676
CAS No.:73202-52-5
- 2''-O-E-p-Coumaroylafzelin
Catalog No.:BCN0689
CAS No.:151455-10-6
- Platanoside
Catalog No.:BCN0690
CAS No.:133740-25-7
- Morelloflavone
Catalog No.:BCN0691
CAS No.:16851-21-1
- Tamarixin
Catalog No.:BCN0692
CAS No.:27542-39-8
- Quercetin 4'-O-galactoside
Catalog No.:BCN0693
CAS No.:381728-34-3
- E,Z-Platanoside
Catalog No.:BCN0694
CAS No.:1197343-17-1
- Kayaflavone
Catalog No.:BCN0695
CAS No.:481-45-8
- (2S)-Pinocembrin 7-O-[2''-O-(5'''-O-trans-cinnamoyl)-β-D-apiofuranosyl]-β-D-glucoside
Catalog No.:BCN0696
CAS No.:773899-29-9
- Luteolin 5,3'-dimethyl ether
Catalog No.:BCN0697
CAS No.:62346-14-9
- Violanone
Catalog No.:BCN0698
CAS No.:52250-38-1
- Luteolin 5-methyl ether
Catalog No.:BCN0699
CAS No.:58115-29-0
- Delicaflavone
Catalog No.:BCN0700
CAS No.:343569-15-3
Protease-inhibiting, molecular modeling and antimicrobial activities of extracts and constituents from Helichrysum foetidum and Helichrysum mechowianum (compositae).[Pubmed:26042155]
Chem Cent J. 2015 May 30;9:32.
BACKGROUND: Helichrysum species are used extensively for stress-related ailments and as dressings for wounds normally encountered in circumcision rites, bruises, cuts and sores. It has been reported that Helichysum species are used to relief abdominal pain, heart burn, cough, cold, wounds, female sterility, menstrual pain. RESULTS: From the extracts of Helichrysum foetidum (L.) Moench, six known compounds were isolated and identified. They were 7, 4'-dihydroxy-5-methoxy-flavanone (1), 6'-methoxy-2',4, 4'-trihydroxychalcone (2), 6'-methoxy-2',4-dihydroxychalcone -4'-O-beta-D-glucoside (3), apigenin (4), apigenin-7-O-beta-D-glucoside (5), kaur-16-en-18-oic acid (6) while two known compounds 3,5,7-Trihydroxy-8-methoxyflavone (12), 4,5-dicaffeoyl quinic acid (13) together with a mixture of phytosterol were isolated from the methanol extract of Helichrysum mechowianum Klatt. All the compounds were characterized by spectroscopic and mass spectrometric methods, and by comparison with literature data. Both extracts and all the isolates were screened for the protease inhibition, antibacterial and antifungal activities. In addition, the phytochemical profiles of both species were investigated by ESI-MS experiments. CONCLUSIONS: These results showed that the protease inhibition assay of H. foetidum could be mainly attributed to the constituents of flavonoids glycosides (3, 5) while the compound (13) from H. mechowianum contributes to the stomach protecting effects. In addition, among the antibacterial and antifungal activities of all the isolates, compound (6) was found to possess a potent inhibitor effect against the tested microorganisms. The heterogeneity of the genus is also reflected in its phytochemical diversity. The differential bioactivities and determined constituents support the traditional use of the species. Molecular modelling was carried out by computing selected descriptors related to drug absorption, distribution, metabolism, excretion and toxicity (ADMET). Graphical abstractCompounds isolated from Helichrysum species (Compositae).
Isolation and identification of antibacterial and cytotoxic compounds from the leaves of Muntingia calabura L.[Pubmed:23276785]
J Ethnopharmacol. 2013 Mar 7;146(1):198-204.
ETHNOPHARMACOLOGICAL RELEVANCE: Muntingia calabura (Elaeocarpaceae) is one of the most common roadside trees in Malaysia. Its leaves, barks, flowers and roots have been used as a folk remedy for the treatment of fever, incipient cold, liver disease, as well as an antiseptic agent in Southeast Asia. The aim of this study is to isolate and identify the antibacterial and cytotoxic compounds from the leaves of Muntingia calabura L. MATERIALS AND METHODS: Antibacterial and cytotoxic activities were determined by micro-broth dilution and MTT assays, respectively. Seven fractions (F1-F7), three flavones and a chalcone were isolated from the active EtOAc extract using bioassay-guided screening. The structures of four compounds were elucidated by spectroscopic methods and compared with published data. The compounds were further tested for their antibacterial and cytotoxic activities. RESULTS: Three flavones and a chalcone [5,7-dihydroxy-3,8-dimethoxyflavone (1), 2',4'-dihydroxychalcone (2), 5-hydroxy-3,7-dimethoxyflavone (3) and 3,5,7-Trihydroxy-8-methoxyflavone (4)] were isolated from the active fraction F5 of EtOAc extract. Compounds 1 and 3 were isolated for the first time from Muntingia calabura L. Antibacterial activity indicates that compound 2 exhibited the most significant activity with MIC value of 50 mug/mL and 100 mug/mL against MSSA and MRSA, respectively. Cytotoxic activity indicates that compounds 2 and 3 exhibited very strong activity against HL60 with IC50 values of 3.43 mug/mL and 3.34 mug/mL, respectively. CONCLUSION: The antibacterial activity of the leaves of Muntingia calabura L. is ascribable to the active compound 2 while the cytotoxic activity is ascribable to the active compounds 2 and 3.


