Luteolin 5-methyl etherCAS# 58115-29-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 58115-29-0 | SDF | Download SDF |
| PubChem ID | 13964550 | Appearance | Yellow powder |
| Formula | C16H12O6 | M.Wt | 300.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
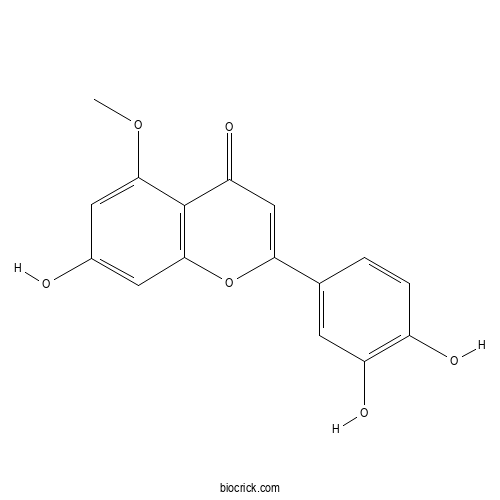
| Chemical Name | 2-(3,4-dihydroxyphenyl)-7-hydroxy-5-methoxychromen-4-one | ||
| SMILES | COC1=CC(=CC2=C1C(=O)C=C(O2)C3=CC(=C(C=C3)O)O)O | ||
| Standard InChIKey | OZPMKAZMPNDLKX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12O6/c1-21-14-5-9(17)6-15-16(14)12(20)7-13(22-15)8-2-3-10(18)11(19)4-8/h2-7,17-19H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Luteolin 5-methyl ether Dilution Calculator

Luteolin 5-methyl ether Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.33 mL | 16.65 mL | 33.3 mL | 66.6001 mL | 83.2501 mL |
| 5 mM | 0.666 mL | 3.33 mL | 6.66 mL | 13.32 mL | 16.65 mL |
| 10 mM | 0.333 mL | 1.665 mL | 3.33 mL | 6.66 mL | 8.325 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.332 mL | 1.665 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.666 mL | 0.8325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Violanone
Catalog No.:BCN0698
CAS No.:52250-38-1
- Luteolin 5,3'-dimethyl ether
Catalog No.:BCN0697
CAS No.:62346-14-9
- (2S)-Pinocembrin 7-O-[2''-O-(5'''-O-trans-cinnamoyl)-β-D-apiofuranosyl]-β-D-glucoside
Catalog No.:BCN0696
CAS No.:773899-29-9
- Kayaflavone
Catalog No.:BCN0695
CAS No.:481-45-8
- E,Z-Platanoside
Catalog No.:BCN0694
CAS No.:1197343-17-1
- Quercetin 4'-O-galactoside
Catalog No.:BCN0693
CAS No.:381728-34-3
- Tamarixin
Catalog No.:BCN0692
CAS No.:27542-39-8
- Morelloflavone
Catalog No.:BCN0691
CAS No.:16851-21-1
- Platanoside
Catalog No.:BCN0690
CAS No.:133740-25-7
- 2''-O-E-p-Coumaroylafzelin
Catalog No.:BCN0689
CAS No.:151455-10-6
- 3,5,7-Trihydroxy-8-methoxyflavone
Catalog No.:BCN0688
CAS No.:5928-42-7
- 8-(1,1-Dimethyl-2-propenyl)kaempferol
Catalog No.:BCN0687
CAS No.:142646-43-3
- Delicaflavone
Catalog No.:BCN0700
CAS No.:343569-15-3
- 5,6-Dihydroxy-7,8-dimethoxyflavone
Catalog No.:BCN0701
CAS No.:76844-65-0
- Shanciol B
Catalog No.:BCN0702
CAS No.:208106-53-0
- Ochnaflavone
Catalog No.:BCN0703
CAS No.:50276-96-5
- 3',3'''-Biapigenin
Catalog No.:BCN0704
CAS No.:151455-26-4
- Chrysocauloflavone I
Catalog No.:BCN0705
CAS No.:899789-51-6
- 6,7,4'-Trihydroxyflavanone
Catalog No.:BCN0706
CAS No.:189689-31-4
- Procyanidin B2 3,3'-di-O-gallate
Catalog No.:BCN0707
CAS No.:79907-44-1
- 2'',3''-Dihydro-3',3'''-biapigenin
Catalog No.:BCN0708
CAS No.:151455-25-3
- 3',3'''-Binaringenin
Catalog No.:BCN0709
CAS No.:145399-99-1
- 2'',3''-Dihydroochnaflavone
Catalog No.:BCN0710
CAS No.:340997-02-6
- Procyanidin B5
Catalog No.:BCN0711
CAS No.:12798-57-1
Phytochemical Investigation on Volatile Compositions and Methoxylated Flavonoids of Agrostis gigantea Roth.[Pubmed:33224243]
Iran J Pharm Res. 2020 Spring;19(2):360-370.
In this study, methoxylated flavonoids and volatile constitutions of Agrostis gigantea Roth (Poaceae) were investigated for the first time. The flavonoids were identified by spectroscopic methods ((1)H-NMR, (13)C-NMR, COSY, NOSEY, TCOSY, and HMBC). The volatile constitutions of aerial parts and seeds were analyzed by gas chromatography-mass spectrometry (GC-MS). Two methoxylated flavonoids, Luteolin 5-methyl ether (1), and cirsilineol (2) were isolated from the aerial parts of this plant. According to the GC-MS data the main constitutions of these volatile oils belong to the simple phenolic category which include coniferyl alcohol (18.80%) and eugenol (12.19%) in aerial parts and seeds, respectively. By using the computer- aided molecular modeling approaches, the binding affinity of these compounds was predicted in the catalytic domains of aryl hydrocarbon receptor (AhR). These two isolated flavonoids were investigated in-vitro for their inhibitory activity on 4T1 breast carcinoma cells. It was predicted that these compounds could be well-matched in aryl hydrocarbon receptor (3H82) active site, but based on the in-vitro assay, the IC50 values on cytotoxicity were 428.24 +/-3.21 and 412.7+/-3.02 mug/mL for Luteolin 5-methyl ether and cirsilineol, respectively. Thus, it can be concluded that these flavonoids exhibit low cytotoxicity against 4T1 breast carcinoma cell line.
A new furofuran lignan diglycoside and other secondary metabolites from the antidepressant extract of Castilleja tenuiflora Benth.[Pubmed:26197306]
Molecules. 2015 Jul 21;20(7):13127-43.
Castilleja tenuiflora has been used for the treatment of several Central Nervous System (CNS) diseases. Herein we report the antidepressant activity of the methanol extract from the leaves of this medicinal plant. The oral administration of MeOH extract (500 mg/kg) induced a significant (p < 0.05) decrement of the immobility parameter on Forced Swimming Test (FST) and an increment in the latency and duration of the hypnosis, induced by administration of sodium pentobarbital (Pbi, 40 mg/kg, i.p.). Chemical analysis of this antidepressant extract allowed the isolation of (+)-piperitol-4-O-xylopyranosyl-(1-->6)-O-glucopyranoside. This new furofuran lignan diglycoside was named tenuifloroside (1) and its complete chemical structure elucidation on the basis of 1D and 2D NMR spectra analysis of the natural compound 1 and its peracetylated derivative 1a is described. This compound was found together with two flavones-apigenin and Luteolin 5-methyl ether-a phenylethanoid-verbascoside-and three iridoids-geniposide, caryoptoside and aucubin. All these compounds were purified by successive normal and reverse phase column chromatography. Tenuifloroside, caryoptoside and Luteolin 5-methyl ether were isolated from Castilleja genus for the first time. These findings demonstrate that C. tenuiflora methanol extract has beneficial effect on depressive behaviors, and the knowledge of its chemical constitution allows us to propose a new standardized treatment for future investigations of this species in depressive illness.
Chemical constituents isolated from Juncus effusus induce cytotoxicity in HT22 cells.[Pubmed:25794817]
J Nat Med. 2015 Jul;69(3):421-6.
Effususol A (1), a new 9,10-dihydrophenanthrene, has been isolated from the medullae of Juncus effusus along with ten known compounds, effusol (2), dehydroeffusol (3), juncusol (4), dehydrojuncusol (5), juncuenin B (6), dehydrojuncuenin B (7), juncuenin D (8), luteolin (9), Luteolin 5-methyl ether (10), and 4-hydroxy-2,3-dimethyl-2-nonen-4-olide (11). The structure of 1 was elucidated on the basis of spectroscopic data. 2, 4, 6, 7, and 8 have induced caspase-3-mediated cytotoxicity in HT22 cells.


