OchnaflavoneCAS# 50276-96-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 50276-96-5 | SDF | Download SDF |
| PubChem ID | 5492110 | Appearance | Yellow powder |
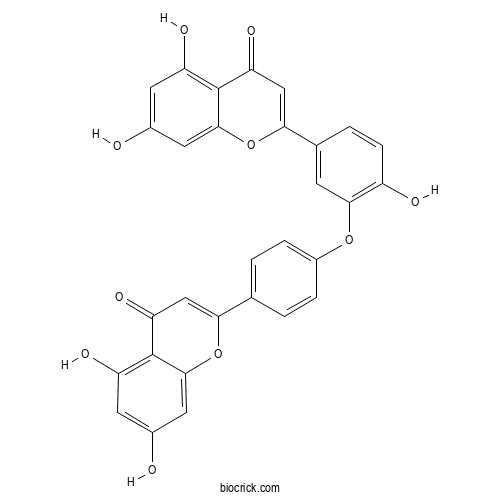
| Formula | C30H18O10 | M.Wt | 538.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-[4-[5-(5,7-dihydroxy-4-oxochromen-2-yl)-2-hydroxyphenoxy]phenyl]-5,7-dihydroxychromen-4-one | ||
| SMILES | C1=CC(=CC=C1C2=CC(=O)C3=C(C=C(C=C3O2)O)O)OC4=C(C=CC(=C4)C5=CC(=O)C6=C(C=C(C=C6O5)O)O)O | ||
| Standard InChIKey | NNPGECDACGBKDH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H18O10/c31-16-8-20(34)29-22(36)12-24(39-27(29)10-16)14-1-4-18(5-2-14)38-26-7-15(3-6-19(26)33)25-13-23(37)30-21(35)9-17(32)11-28(30)40-25/h1-13,31-35H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ochnaflavone Dilution Calculator

Ochnaflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.857 mL | 9.2851 mL | 18.5701 mL | 37.1402 mL | 46.4253 mL |
| 5 mM | 0.3714 mL | 1.857 mL | 3.714 mL | 7.428 mL | 9.2851 mL |
| 10 mM | 0.1857 mL | 0.9285 mL | 1.857 mL | 3.714 mL | 4.6425 mL |
| 50 mM | 0.0371 mL | 0.1857 mL | 0.3714 mL | 0.7428 mL | 0.9285 mL |
| 100 mM | 0.0186 mL | 0.0929 mL | 0.1857 mL | 0.3714 mL | 0.4643 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Shanciol B
Catalog No.:BCN0702
CAS No.:208106-53-0
- 5,6-Dihydroxy-7,8-dimethoxyflavone
Catalog No.:BCN0701
CAS No.:76844-65-0
- Delicaflavone
Catalog No.:BCN0700
CAS No.:343569-15-3
- Luteolin 5-methyl ether
Catalog No.:BCN0699
CAS No.:58115-29-0
- Violanone
Catalog No.:BCN0698
CAS No.:52250-38-1
- Luteolin 5,3'-dimethyl ether
Catalog No.:BCN0697
CAS No.:62346-14-9
- (2S)-Pinocembrin 7-O-[2''-O-(5'''-O-trans-cinnamoyl)-β-D-apiofuranosyl]-β-D-glucoside
Catalog No.:BCN0696
CAS No.:773899-29-9
- Kayaflavone
Catalog No.:BCN0695
CAS No.:481-45-8
- E,Z-Platanoside
Catalog No.:BCN0694
CAS No.:1197343-17-1
- Quercetin 4'-O-galactoside
Catalog No.:BCN0693
CAS No.:381728-34-3
- Tamarixin
Catalog No.:BCN0692
CAS No.:27542-39-8
- Morelloflavone
Catalog No.:BCN0691
CAS No.:16851-21-1
- 3',3'''-Biapigenin
Catalog No.:BCN0704
CAS No.:151455-26-4
- Chrysocauloflavone I
Catalog No.:BCN0705
CAS No.:899789-51-6
- 6,7,4'-Trihydroxyflavanone
Catalog No.:BCN0706
CAS No.:189689-31-4
- Procyanidin B2 3,3'-di-O-gallate
Catalog No.:BCN0707
CAS No.:79907-44-1
- 2'',3''-Dihydro-3',3'''-biapigenin
Catalog No.:BCN0708
CAS No.:151455-25-3
- 3',3'''-Binaringenin
Catalog No.:BCN0709
CAS No.:145399-99-1
- 2'',3''-Dihydroochnaflavone
Catalog No.:BCN0710
CAS No.:340997-02-6
- Procyanidin B5
Catalog No.:BCN0711
CAS No.:12798-57-1
- Procyanidin B2 3''-O-gallate
Catalog No.:BCN0712
CAS No.:73086-04-1
- 2"-O-Glucosylrutin
Catalog No.:BCN0713
CAS No.:55696-55-4
- 2,3-Dihydroamentoflavone
Catalog No.:BCN0714
CAS No.:34340-51-7
- Procyanidin B-5 3,3'-di-O-gallate
Catalog No.:BCN0715
CAS No.:106533-60-2
Phytochemical profile of Cespedesia spathulata leaves (Ochnaceae) and its effect on tyrosinase enzyme.[Pubmed:34495202]
An Acad Bras Cienc. 2021 Sep 3;93(4):e20200443.
Phytochemical studies of Cespedesia spathulata (Ochnaceae) leaves using 1H, 13C NMR, and GC-MS have led to the isolation of some metabolites identified for the first time in these species such as cathechin, epicatechin, vitexin, orientin, 6''-O-acetyl-vitexin, sitosterol, stigmasterol, phytol, 4,5-dihydrovomifoliol and a mixture of aliphatic methyl esters, together with Ochnaflavone, which was previously isolated from this plant. The modulating activity of some fractions and compounds from Cespedesia spathulata towards tyrosinase enzyme was assayed by spectroscopic and theoretical means/experiments. The dichloromethane fraction (133 mug mL-1) and Ochnaflavone (333 muM) inhibited tyrosinase activity by 20 % and 2.0 %, respectively, whereas the ethyl acetate fraction (666 mug mL-1) and +/-catechins (catechin and epicatechin - 800 muM) activated it by 104 % and 384 %, respectively. Quantum chemical calculations suggested that catechin and epicatechin are better activators than L-DOPA by interacting with Cu (II) ions. Molecular docking results suggested that hydrogen bonding and hydrophobic interactions are the main binding forces between each tyrosinase activator and the amino acid residues inside the active protein binding pocket.
Polypharmacology of some medicinal plant metabolites against SARS-CoV-2 and host targets: Molecular dynamics evaluation of NSP9 RNA binding protein.[Pubmed:34370622]
J Biomol Struct Dyn. 2021 Aug 9:1-17.
Medicinal plants as rich sources of bioactive compounds are now being explored for drug development against COVID-19. 19 medicinal plants known to exhibit antiviral and anti-inflammatory effects were manually curated, procuring a library of 521 metabolites; this was virtually screened against NSP9, including some other viral and host targets and were evaluated for polypharmacological indications. Leads were identified via rigorous scoring thresholds and ADMET filtering. MM-GBSA calculation was deployed to select NSP9-Lead complexes and the complexes were evaluated for their stability and protein-ligand communication via MD simulation. We identified 5 phytochemical leads for NSP9, 23 for Furin, 18 for ORF3a, and 19 for IL-6. Ochnaflavone and Licoflavone B, obtained from Lonicera japonica (Japanese Honeysuckle) and Glycyrrhiza glabra (Licorice), respectively, were identified to have the highest potential polypharmacological properties for the aforementioned targets and may act on multiple pathways simultaneously to inhibit viral entry, replication, and disease progression. Additionally, MD simulation supports the robust stability of Ochnaflavone and Licoflavone B against NSP9 at the active sites via hydrophobic interactions, H-bonding, and H-bonding facilitated by water. This study promotes the initiation of further experimental analysis of natural product-based anti-COVID-19 therapeutics. Communicated by Ramaswamy H. Sarma.
Hinokiflavone and Related C-O-C-Type Biflavonoids as Anti-cancer Compounds: Properties and Mechanism of Action.[Pubmed:33534099]
Nat Prod Bioprospect. 2021 Aug;11(4):365-377.
Biflavonoids are divided in two classes: C-C type compounds represented by the dimeric compound amentoflavone and C-O-C-type compounds typified by hinokiflavone (HNK) with an ether linkage between the two connected apigenin units. This later sub-group of bisflavonyl ethers includes HNK, Ochnaflavone, delicaflavone and a few other dimeric compounds, found in a variety of plants, notably Selaginella species. A comprehensive review of the anticancer properties and mechanism of action of HNK is provided, to highlight the anti-proliferative and anti-metastatic activities of HNK and derivatives, and HNK-containing plant extracts. The anticancer effects rely on the capacity of HNK to interfere with the ERK1-2/p38/NFkappaB signaling pathway and the regulation of the expression of the matrix metalloproteinases MMP-2 and MMP-9 (with a potential direct binding to MMP-9). In addition, HNK was found to function as a potent modulator of pre-mRNA splicing, inhibiting the SUMO-specific protease SENP1. As such, HNK represents a rare SENP1 inhibitor of natural origin and a scaffold to design synthetic compounds. Oral formulations of HNK have been elaborated to enhance its solubility, to facilitate the compound delivery and to enhance its anticancer efficacy. The review shed light on the anticancer potential of C-O-C-type biflavonoids and specifically on the pharmacological profile of HNK. This compound deserves further attention as a regulator of pre-mRNA splicing, useful to treat cancers (in particular hepatocellular carcinoma) and other human pathologies.
Natural biflavonoids as potential therapeutic agents against microbial diseases.[Pubmed:33493916]
Sci Total Environ. 2021 May 15;769:145168.
Microbes broadly constitute several organisms like viruses, protozoa, bacteria, and fungi present in our biosphere. Fast-paced environmental changes have influenced contact of human populations with newly identified microbes resulting in diseases that can spread quickly. These microbes can cause infections like HIV, SARS-CoV2, malaria, nosocomial Escherichia coli, methicillin-resistant Staphylococcus aureus (MRSA), or Candida infection for which there are no available vaccines/drugs or are less efficient to prevent or treat these infections. In the pursuit to find potential safe agents for therapy of microbial infections, natural biflavonoids like amentoflavone, tetrahydroamentoflavone, ginkgetin, bilobetin, morelloflavone, agathisflavone, hinokiflavone, Garcinia biflavones 1 (GB1), Garcinia biflavones 2 (GB2), robustaflavone, strychnobiflavone, Ochnaflavone, dulcisbiflavonoid C, tetramethoxy-6,6''-bigenkwanin and other derivatives isolated from several species of plants can provide effective starting points and become a source of future drugs. These biflavonoids show activity against influenza, severe acute respiratory syndrome (SARS), dengue, HIV-AIDS, coxsackieviral, hepatitis, HSV, Epstein-Barr virus (EBV), protozoal (Leishmaniasis, Malaria) infections, bacterial and fungal infections. Some of the biflavonoids can provide antiviral and protozoal activity by inhibition of neuraminidase, chymotrypsin-like protease, DV-NS5 RNA dependant RNA polymerase, reverse transcriptase (RT), fatty acid synthase, DNA polymerase, UL54 gene expression, Epstein-Barr virus early antigen activation, recombinant cysteine protease type 2.8 (r-CPB2.8), Plasmodium falciparum enoyl-acyl carrier protein (ACP) reductase or cause depolarization of parasitic mitochondrial membranes. They may also provide anti-inflammatory therapeutic activity against the infection-induced cytokine storm. Considering the varied bioactivity of these biflavonoids against these organisms, their structure-activity relationships are derived and wherever possible compared with monoflavones. Overall, this review aims to highlight these natural biflavonoids and briefly discuss their sources, reported mechanism of action, pharmacological uses, and comment on resistance mechanism, flavopiridol repurposing and the bioavailability aspects to provide a starting point for anti-microbial research in this area.
Anti-inflammatory property and functional substances of Lonicerae Japonicae Caulis.[Pubmed:33189843]
J Ethnopharmacol. 2021 Mar 1;267:113502.
ETHNOPHARMACOLOGICAL RELEVANCE: Lonicerae Japonicae Caulis, the dried stem and branch of Lonicera japonica Thunb., is a Chinese Materia Medica known as Ren Dong Teng in Chinese with long use history in the traditional Chinese medicine (TCM) prescriptions. Lonicerae Japonicae Caulis possesses heat-clearing and detoxifying functions according to the TCM theory. In recent years, a large amount of experimental and clinical studies proved good anti-inflammatory effects of some heat-clearing and detoxifying herbs. The present study aims to reveal the anti-inflammatory property and functional substances of Lonicerae Japonicae Caulis. MATERIALS AND METHODS: For anti-inflammatory activity test, LPS-induced RAW 264.7 macrophages, DSS-induced SPF male C57BL/6J mice model, and LPS-induced SPF male ICR mice model were used in vitro and in vivo, respectively. The behavioral changes, organ damage, and the expression of inflammatory factors such as TNT-alpha and IL-6 mRNA expression were measured for activity evaluation. Lonicerae Japonicae Caulis samples were prepared by solvent extraction and subsequent column chromatography. The main components were identified and determined using UPLC-UV analysis as well as NMR interpretation after purification. To testify the contribution of main components for the anti-inflammatory activity, different samples were also prepared by compound-knockout strategy. RESULTS: Ethanol extract of Lonicerae Japonicae Caulis could attenuate sickness symptoms in mice such as diarrhea, less activity, and depression. It could also alleviate multiple organ damage, and significantly inhibit the expression of pro-inflammatory factors such as TNF-alpha, IL-1beta, IL-6 and IFN-gamma in mice. Furthermore, the isochlorogenic acid-rich and biflavonoid-rich fractions and isochlorogenic acids A and C, and Ochnaflavone could significantly down-regulate the mRNA expression of TNF-alpha and IL-6 in LPS-induced RAW 264.7 macrophages. CONCLUSIONS: Lonicerae Japonicae Caulis possesses anti-inflammatory property. Its isochlorogenic acid-rich and biflavonoid-rich fractions do the major contribution. And their main components, isochlorogenic acids A and C, and Ochnaflavone, take main responsibility for the anti-inflammatory property.
Secondary metabolites from Triclisia gilletii (De Wild) Staner (Menispermaceae) with antimycobacterial activity against Mycobacterium tuberculosis.[Pubmed:29144174]
Nat Prod Res. 2019 Mar;33(5):642-650.
Triclisinone (2), a new Ochnaflavone derivative, was isolated from the aerial parts of Triclisia gilletii, along with known drypemolundein B (1) and eight other known compounds. The chemical shifts of drypemolundein B (1) have been partially revised based on reinterpretation of NMR spectroscopic data. The eight other secondary metabolites are composed of: (+)-nonacosan-10-ol (3); stigmasterol (4), 3-O-beta-D-glucopyranosylsitosterol (5), 3-O-beta-D-glucopyranosylstigmasterol (6); oleanic acid (7); myricetin (8), quercetin (9) and 3-methoxyquercetin (10). Their structures were elucidated using IR, MS, NMR 1D and 2D, (1)H and (13)C and comparison with literature data. Furthermore, compounds 1, 2, 5, 6, 8, 9 and the crude extract were tested against Mycobacterium tuberculosis. Compounds 1, 2, 8 and 9 displayed moderate to very good activity against resistant strain (codified AC 45) of M. tuberculosis with minimum inhibitory concentrations MICs ranging from 3.90 to 62.5 mug/mL.
A Nitrile Glucoside and Biflavones from the Leaves of Campylospermum excavatum (Ochnaceae).[Pubmed:28695668]
Chem Biodivers. 2017 Nov;14(11).
The study of the MeOH extract of the leaves of Campylospermum excavatum led to the isolation of a nitrile glucoside, named campyloside C (1) and an original derivative of Ochnaflavone, 7-O-methylOchnaflavone (2), along with three known biflavonoids, amentoflavone, sequoiaflavone, and sotetsuflavone (3 - 5). The linkage site of the sub-units of 2 was confirmed by chemical correlation, after semi-synthesis of a trimethoxylated derivative of Ochnaflavone (2a). The structures of these compounds as well as their relative and absolute configurations were assigned by 1D- and 2D-NMR experiments, HR-ESI-MS and Electronic Circular Dichroism (ECD) calculations. A low-pass J filter HMBC experiment was performed in order to define the configuration of the double bond of 1. All of the biflavonoids were evaluated against protozoan parasites. Amentoflavone moderately inhibited the promastigote form of Leishmania infantum.
Anti-proliferative study and isolation of Ochnaflavone from the ethyl acetate-soluble fraction of Ochna kibbiensis Hutch & Dalziel.[Pubmed:28032512]
Nat Prod Res. 2017 Sep;31(18):2149-2152.
Anti-proliferative activity of the ethyl acetate fractions of Ochna schweinfurthiana F. Hoffm. and Ochna kibbiensis Hutch. and Dalziel methanol leaf extracts were investigated against glioblastoma multiforme (GBM U-1242 MG) cell line. O. kibbiensis significantly (p < 0.001) and dose dependently (IC50 = 25.74 mug/mL) reduced cell count. At 125 mug/mL, O. kibbiensis extract reduced cell count by about 92% compared to the untreated control. On the other hand, at 125 mug/mL, O. schweinfurthiana extract reduced cell count only by 20%, indicating a much weaker activity (IC50 = 823.51 mug/mL). Following from the result obtained, ethyl acetate fraction of O. kibbiensis was subjected to chromatographic purification. This led to the isolation of Ochnaflavone; the structure of the isolated compound was identified by analysis of its nuclear magnetic resonance (NMR) spectral data and comparison with data in the literature. Although the isolated Ochnaflavone could not be tested for anti-proliferative activity due to insufficient quantity, the obtained results indicate the presence of bioactive anti-GBM principles in O. kibbiensis.
Total synthesis of ochnaflavone.[Pubmed:23946830]
Beilstein J Org Chem. 2013 Jul 8;9:1346-51.
The first total syntheses of Ochnaflavone, an asymmetric biflavone consisting of apigenin and luteolin moieties, and the permethyl ether of 2,3,2'',3''-tetrahydroOchnaflavone have been achieved. The key steps in the synthesis of Ochnaflavone were the formation of a diaryl ether and ring cyclization of an ether-linked dimeric chalcone to assemble the two flavone nuclei. Optimal experimental conditions for the oxidative cyclization to form Ochnaflavone were established.
Ochnaflavone and ochnaflavone 7-O-methyl ether two antibacterial biflavonoids from Ochna pretoriensis (Ochnaceae).[Pubmed:23413563]
Nat Prod Commun. 2012 Dec;7(12):1601-4.
The acetone extract of Ochna pretoriensis was evaluated for antibacterial activity using bioautography and serial microplate dilution methods against four nosocomial bacterial pathogens namely: Escherichia coli, Staphylococcus aureus, Enterococcus faecalis and Pseudomonas aeruginosa. A bioassay-guided fractionation of the crude extract led to the isolation of two antibacterial biflavonoids, Ochnaflavone and Ochnaflavone 7-O-methyl ether. Gram-negative bacteria were more sensitive to the isolated compounds than the Gram-positive bacteria (MIC values: 31.3 microg/mL for P. aeruginosa and 62.5 microg/mL for S. aureus). In addition, the isolated compounds were assessed for their potential toxic effects in the MTT toxicity assay using monkey kidney vero cells and Ames genotoxicity test using Salmonella typhimurium strain TA98. LC50 values were 125.9 microg/mL for Ochnaflavone and 162.0/microg/mL for Ochnaflavone 7-O-methyl ether. The isolated compounds have selectivity index values ranging from 1.29 to 4.03. Selectivity index values higher than one indicate that test samples are less toxic to the host cells than to the pathogens. The biflavonoids did not have any mutagenic effects in the Salmonella/microsome assay without metabolic activation.
Involvement of T-cell immunoregulation by ochnaflavone in therapeutic effect on fungal arthritis due to Candida albicans.[Pubmed:21811929]
Arch Pharm Res. 2011 Jul;34(7):1209-17.
Arthritis due to pathogenic fungi is a serious disease causing rapid destruction of the joint. In the pathogenesis of arthritis, T lymphocytes are considered to be one of the major immune cells. In present study, we examined the T cell immunoregulatory effect by Ochnaflavone (Och), a biflavonoid, on arthritis caused by Candida albicans that is the most commonly associated with fungal arthritis. To examine the effects of ochnaflavonon Candida albicans-caused septic arthritis, an emulsified mixture of C. albicans cell wall and complete Freund's adjuvant (CACW/CFA) was injected into BALB/c mice via hind footpad route on days -3, -2, and -1. On Day 0, Och at 1 or 2 mg/dose/time was intratraperitoneally given to mice with the swollen footpad every other day for 3 times. The footpad-edema was measured for 20 days. Results revealed that Och reduced the edema at all dose levels and furthermore, there was app. 45% reduction of the edema in animals given 2 mg-dose at the peak of septic arthritis (p < 0.05). This anti-arthritic effect was accompanied by the diminishing of the DTH (delayed type hypersensitivity) activity against the CACW and by the provoking of the dominant T helper 2 (Th2) type cytokines production (IL-4 and Il-10), which appeared to result in a suppression of T helper 1 cytokines (IFN-gamma and IL-2). Besides the T cell immunoregulatory activity, Och inhibited T cells activation as evidenced by the IL-2 reduction from PMA/ionomycin-stimulated Jurkat cell line and in addition, the compound killed macrophages in a dose-dependent manner (p < 0.05). However, Och caused no hemolysis (p < 0.05). These data implicate that Och, which has anti-arthritic activity based on the Th2 dominance as well as macrophage removal, can be safely administered into the blood circulation for treatment of the arthritis caused by C. albicans. Thus, it can be concluded that Och would be an ideal immunologically evaluated agent for treating of Candida arthritis.
Inhibition of arachidonate release from rat peritoneal macrophage by biflavonoids.[Pubmed:18982255]
Arch Pharm Res. 1997 Dec;20(6):533-8.
Biflavonoid is one of unique classes of naturally-occurring bioflavonoid. Previously, certain biflavonoids were found to possess the inhibitory effects on phospholipase A(2) activity and lymphocytes proliferation(1) suggesting their anti-inflammatory/immunoregulatory potential. In this study, effects of several biflavonoids on arachidonic acid release from rat peritoneal macrophages were investigated, because arachidonic acid released from the activated macrophages is one of the indices of inflammatory conditions. When resident peritoneal macrophages labeled with [(3)H]arachidonic acid were activated by phorbol 12-myristate 13-acetate (PMA) or calcium ionophore, A23187, radioactivity released in the medium was increased approximately 4.1 approximately 7.3 fold after 120 min incubation compared to the spontaneous release in the control incubation. In this condition, biflavonoids (10 uM) such as Ochnaflavone, ginkgetin and isoginkgetin, showed inhibition of arachidonate release from macrophages activated by PMA (32.5 approximately 40.0% inhibition) or A23187 (21.7 approximately 41.7% inhibition). Amentoflavone showed protection only against PMA-induced arachidonate release, while apigenin, a monomer of these biflavonoids, did not show the significant inhibition up to 10 uM. Staurosporin (1 uM), a protein kinase C inhibitor, showed an inhibitory effect only against PMA-induced arachidonate release (96.8% inhibition). Inhibition of arachidonate release from the activated macrophages may contribute to an anti-inflammatory potential of biflavonoidsin vivo.
Biochemical pharmacology of biflavonoids: implications for anti-inflammatory action.[Pubmed:18409037]
Arch Pharm Res. 2008 Mar;31(3):265-73.
Biflavonoids belong to a subclass of the plant flavonoid family. Distribution of biflavonoids in the plant kingdom is limited to several species. Previously, some pharmacological activities of biflavonoids were described such as inhibition of histamine release from mast cells and inhibition of lymphocyte proliferation, suggesting the anti-inflammatory/antiallergic potential of the biflavonoids. Furthermore, several natural biflavonoids including Ochnaflavone and ginkgetin inhibit phospholipase A2. Most importantly, certain biflavonoids exhibit anti-inflammatory activity through the regulation of proinflammatory gene expression in vitro and in vivo. Recently, several synthetic approaches yielded new biflavonoid molecules with anti-inflammatory potential. These molecules also exhibit phospholipase A2 and cyclooxygenase-2 inhibitory activity. Although the bioavailability needs be improved, certain biflavonoids may have potential as new anti-inflammatory agents. This is the first review of biflavonoid pharmacology to date.
Anti HIV-1 flavonoid glycosides from Ochna integerrima.[Pubmed:17562490]
Planta Med. 2007 Jun;73(7):683-8.
Bioassay-guided fractionation of the anti-HIV-1 active EtOAc extract from leaves and twigs of Ochna integerrima led to the isolation of five new flavonoid glycosides 1 - 5, five known flavonoids 6 - 10, and two known flavonoid glycosides 11 and 12. Structures were determined based on spectroscopic analyses. 6- gamma, gamma-Dimethylallyldihydrokaempferol 7- O- beta-D-glucoside (1), 6-gamma, gamma-dimethylallylquercetin 7- O- beta- D-glucoside (3), 6-(3-hydroxy-3-methylbutyl)taxifolin 7- O- beta-D-glucoside (4), 6-(3-hydroxy-3-methylbutyl)quercetin 7- O-beta-D-glucoside (5), and 6-gamma, gamma-dimethylallyltaxifolin 7-O-beta-D-glucoside (11) showed anti-HIV-1 activities in the syncytium assay using the (Delta Tat/rev)MC99 virus and the 1A2 cell line system with EC(50) values ranging from 14.0 - 102.4 microg/mL. Furthermore, Ochnaflavone 7''-O-methyl ether (7) and 2'', 3''-dihydroOchnaflavone 7''-O-methyl ether (8) were very active; they exerted activities in the syncytium assay with EC(50) values of 2.0 and 0.9 microg/mL, respectively, and likewise inhibited HIV-1 reverse transcriptase (RT) with IC(50) values of 2.0 and 2.4 microg/mL, respectively.


