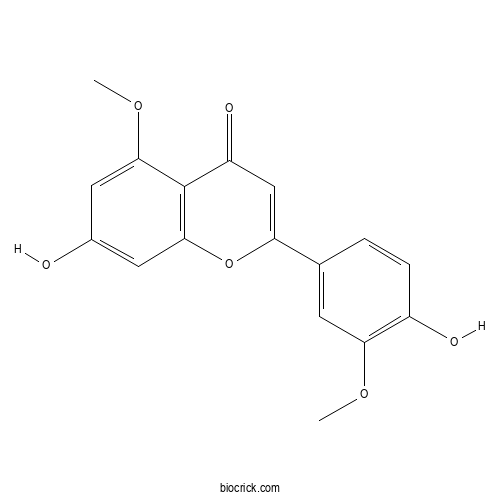
Luteolin 5,3'-dimethyl etherCAS# 62346-14-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 62346-14-9 | SDF | Download SDF |
| PubChem ID | 13964549 | Appearance | Yellow powder |
| Formula | C17H14O6 | M.Wt | 314.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 7,4'-Dihydroxy-5,3'-dimethoxyflavone | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 7-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-5-methoxychromen-4-one | ||
| SMILES | COC1=CC(=CC2=C1C(=O)C=C(O2)C3=CC(=C(C=C3)O)OC)O | ||
| Standard InChIKey | JDMXMMBASFOTIF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14O6/c1-21-14-5-9(3-4-11(14)19)13-8-12(20)17-15(22-2)6-10(18)7-16(17)23-13/h3-8,18-19H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Luteolin 5,3'-dimethyl ether Dilution Calculator

Luteolin 5,3'-dimethyl ether Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1817 mL | 15.9084 mL | 31.8167 mL | 63.6335 mL | 79.5418 mL |
| 5 mM | 0.6363 mL | 3.1817 mL | 6.3633 mL | 12.7267 mL | 15.9084 mL |
| 10 mM | 0.3182 mL | 1.5908 mL | 3.1817 mL | 6.3633 mL | 7.9542 mL |
| 50 mM | 0.0636 mL | 0.3182 mL | 0.6363 mL | 1.2727 mL | 1.5908 mL |
| 100 mM | 0.0318 mL | 0.1591 mL | 0.3182 mL | 0.6363 mL | 0.7954 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (2S)-Pinocembrin 7-O-[2''-O-(5'''-O-trans-cinnamoyl)-β-D-apiofuranosyl]-β-D-glucoside
Catalog No.:BCN0696
CAS No.:773899-29-9
- Kayaflavone
Catalog No.:BCN0695
CAS No.:481-45-8
- E,Z-Platanoside
Catalog No.:BCN0694
CAS No.:1197343-17-1
- Quercetin 4'-O-galactoside
Catalog No.:BCN0693
CAS No.:381728-34-3
- Tamarixin
Catalog No.:BCN0692
CAS No.:27542-39-8
- Morelloflavone
Catalog No.:BCN0691
CAS No.:16851-21-1
- Platanoside
Catalog No.:BCN0690
CAS No.:133740-25-7
- 2''-O-E-p-Coumaroylafzelin
Catalog No.:BCN0689
CAS No.:151455-10-6
- 3,5,7-Trihydroxy-8-methoxyflavone
Catalog No.:BCN0688
CAS No.:5928-42-7
- 8-(1,1-Dimethyl-2-propenyl)kaempferol
Catalog No.:BCN0687
CAS No.:142646-43-3
- Eriodictyol 7-O-methylglucuronide
Catalog No.:BCN0686
CAS No.:133360-42-6
- 8-(1,1-Dimethyl-2-propenyl)-3'-methoxykaempferol
Catalog No.:BCN0685
CAS No.:1859979-00-2
- Violanone
Catalog No.:BCN0698
CAS No.:52250-38-1
- Luteolin 5-methyl ether
Catalog No.:BCN0699
CAS No.:58115-29-0
- Delicaflavone
Catalog No.:BCN0700
CAS No.:343569-15-3
- 5,6-Dihydroxy-7,8-dimethoxyflavone
Catalog No.:BCN0701
CAS No.:76844-65-0
- Shanciol B
Catalog No.:BCN0702
CAS No.:208106-53-0
- Ochnaflavone
Catalog No.:BCN0703
CAS No.:50276-96-5
- 3',3'''-Biapigenin
Catalog No.:BCN0704
CAS No.:151455-26-4
- Chrysocauloflavone I
Catalog No.:BCN0705
CAS No.:899789-51-6
- 6,7,4'-Trihydroxyflavanone
Catalog No.:BCN0706
CAS No.:189689-31-4
- Procyanidin B2 3,3'-di-O-gallate
Catalog No.:BCN0707
CAS No.:79907-44-1
- 2'',3''-Dihydro-3',3'''-biapigenin
Catalog No.:BCN0708
CAS No.:151455-25-3
- 3',3'''-Binaringenin
Catalog No.:BCN0709
CAS No.:145399-99-1
UHPLC-MS phytochemical profiling, biological propensities and in-silico studies of Alhagi maurorum roots: a medicinal herb with multifunctional properties.[Pubmed:32352878]
Drug Dev Ind Pharm. 2020 May;46(5):861-868.
The biological, chemical, and in silico properties of methanol and dichloromethane (DCM) extracts of Alhagi maurorum roots with respect to the antioxidant, enzyme inhibition, and phytochemical composition were evaluated. Total bioactive contents were determined spectrophotometrically, and the individual secondary metabolites composition was assessed via ultra-high-performance liquid chromatography mass spectrometry (UHPLC-MS) analysis. Antioxidant capacities were evaluated using a panoply of assays (2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) free radical scavenging, ferric reducing antioxidant power (FRAP), cupric reducing antioxidant power (CUPRAC), phosphomolybdenum total antioxidant capacity (TAC), and metal chelating activity (MCA)). The enzyme inhibition potential was studied against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), alpha-amylase, alpha-glucosidase, tyrosinase, urease and lipoxygenase (LOX) enzymes. The methanol extract was found to contain higher total phenolic (105.91 mg GAE/g extract) and flavonoid (2.27 mg RE/g extract) contents which can be correlated to its more substantial antioxidant potential as well as AChE, BChE, tyrosinase and alpha-glucosidase inhibition. However, the DCM extract was the most effective against alpha-amylase (1.86 mmol ACAE/g extract) enzyme inhibition. The UHPLC-MS analysis of methanol extract identified the tentative presence of a total of 18 secondary metabolites, including flavonoids, saponins, phenolic and terpenoid derivatives. Three compounds named emmotin A, luteolin 5,3'-dimethyl ether, and preferrugone were further investigated for their in silico molecular docking studies against the tested enzymes. The selected compounds were found to have higher binding interaction with AChE followed by BChE, alpha-glucosidase, alpha-amylase, and tyrosinase. The results of the present study have demonstrated A. mauroram to be considered as a lead source of natural antioxidant and enzyme inhibitor compounds.


