MorelloflavoneCAS# 16851-21-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 16851-21-1 | SDF | Download SDF |
| PubChem ID | 5464454 | Appearance | Yellow powder |
| Formula | C30H20O11 | M.Wt | 556.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
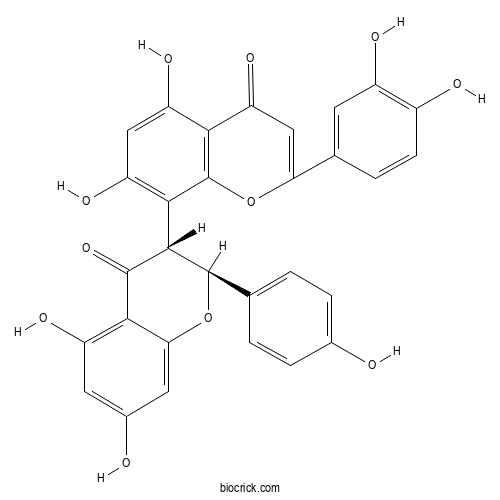
| Chemical Name | 8-[(2S,3R)-5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-2,3-dihydrochromen-3-yl]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one | ||
| SMILES | C1=CC(=CC=C1C2C(C(=O)C3=C(C=C(C=C3O2)O)O)C4=C(C=C(C5=C4OC(=CC5=O)C6=CC(=C(C=C6)O)O)O)O)O | ||
| Standard InChIKey | GFWPWSNIXRDQJC-LMSSTIIKSA-N | ||
| Standard InChI | InChI=1S/C30H20O11/c31-14-4-1-12(2-5-14)29-27(28(39)25-18(35)8-15(32)9-23(25)41-29)26-20(37)10-19(36)24-21(38)11-22(40-30(24)26)13-3-6-16(33)17(34)7-13/h1-11,27,29,31-37H/t27-,29+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Morelloflavone Dilution Calculator

Morelloflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7969 mL | 8.9847 mL | 17.9695 mL | 35.9389 mL | 44.9236 mL |
| 5 mM | 0.3594 mL | 1.7969 mL | 3.5939 mL | 7.1878 mL | 8.9847 mL |
| 10 mM | 0.1797 mL | 0.8985 mL | 1.7969 mL | 3.5939 mL | 4.4924 mL |
| 50 mM | 0.0359 mL | 0.1797 mL | 0.3594 mL | 0.7188 mL | 0.8985 mL |
| 100 mM | 0.018 mL | 0.0898 mL | 0.1797 mL | 0.3594 mL | 0.4492 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Platanoside
Catalog No.:BCN0690
CAS No.:133740-25-7
- 2''-O-E-p-Coumaroylafzelin
Catalog No.:BCN0689
CAS No.:151455-10-6
- 3,5,7-Trihydroxy-8-methoxyflavone
Catalog No.:BCN0688
CAS No.:5928-42-7
- 8-(1,1-Dimethyl-2-propenyl)kaempferol
Catalog No.:BCN0687
CAS No.:142646-43-3
- Eriodictyol 7-O-methylglucuronide
Catalog No.:BCN0686
CAS No.:133360-42-6
- 8-(1,1-Dimethyl-2-propenyl)-3'-methoxykaempferol
Catalog No.:BCN0685
CAS No.:1859979-00-2
- Eriodictyol 7-O-β-D-glucuronide ethyl ester
Catalog No.:BCN0684
CAS No.:847025-48-3
- Naringenin 7-O-β-D-glucuronide methyl ester
Catalog No.:BCN0683
CAS No.:1985597-72-5
- Orientin 2''-O-rhamnoside
Catalog No.:BCN0682
CAS No.:81398-30-3
- 2'-Prenylisorhamnetin
Catalog No.:BCN0681
CAS No.:1932668-04-6
- 6'-Prenylisorhamnetin
Catalog No.:BCN0680
CAS No.:1859979-02-4
- Mikanin
Catalog No.:BCN0679
CAS No.:4324-53-2
- Tamarixin
Catalog No.:BCN0692
CAS No.:27542-39-8
- Quercetin 4'-O-galactoside
Catalog No.:BCN0693
CAS No.:381728-34-3
- E,Z-Platanoside
Catalog No.:BCN0694
CAS No.:1197343-17-1
- Kayaflavone
Catalog No.:BCN0695
CAS No.:481-45-8
- (2S)-Pinocembrin 7-O-[2''-O-(5'''-O-trans-cinnamoyl)-β-D-apiofuranosyl]-β-D-glucoside
Catalog No.:BCN0696
CAS No.:773899-29-9
- Luteolin 5,3'-dimethyl ether
Catalog No.:BCN0697
CAS No.:62346-14-9
- Violanone
Catalog No.:BCN0698
CAS No.:52250-38-1
- Luteolin 5-methyl ether
Catalog No.:BCN0699
CAS No.:58115-29-0
- Delicaflavone
Catalog No.:BCN0700
CAS No.:343569-15-3
- 5,6-Dihydroxy-7,8-dimethoxyflavone
Catalog No.:BCN0701
CAS No.:76844-65-0
- Shanciol B
Catalog No.:BCN0702
CAS No.:208106-53-0
- Ochnaflavone
Catalog No.:BCN0703
CAS No.:50276-96-5
Biflavones from Platonia insignis Mart. Flowers Promote In Vitro Antileishmanial and Immunomodulatory Effects against Internalized Amastigote Forms of Leishmania amazonensis.[Pubmed:34578198]
Pathogens. 2021 Sep 10;10(9). pii: pathogens10091166.
Leishmaniasis is an infectious disease that affects millions of people worldwide, making the search essential for more accessible treatments. The species Platonia insignis Mart. (Clusiaceae) has been extensively studied and has gained prominence for its pharmacological potential. The objective of this work was to evaluate the antileishmania activity, cytotoxic effect and activation patterns of macrophages of hydroalcoholic extract (EHPi), ethyl acetate fractions (FAcOEt) and Morelloflavone/volkensiflavone mixture (MB) from P. insignis flowers. EHPi, FAcOEt and MB demonstrated concentration-dependent antileishmania activity, with inhibition of parasite growth in all analyzed concentrations. EHPi exhibited maximum effect at 800 mug/mL, while FAcOEt and MB reduced the growth of the parasite by 94.62% at 800 mug/mL. EHPi, FAcOEt and MB showed low cytotoxic effects for macrophages at 81.78, 159.67 and 134.28 mug/mL, respectively. EHPi (11.25 microg/mL), FAcOEt (11.25 and 22.5 microg/mL) and MB (22.5 microg/mL) characterized the increase in lysosomal activity, suggesting a possible modulating effect. These findings open for the application of flowers from a P. insignis flowers and biflavones mixture thereof in the promising treatment of leishmaniasis.
Semysinthetic biflavonoid Morelloflavone-7,4',7'',3''',4'''-penta-O-butanoyl is a more potent inhibitor of Proprotein Convertases Subtilisin/Kexin PC1/3 than Kex2 and Furin.[Pubmed:34560176]
Biochim Biophys Acta Gen Subj. 2021 Sep 22;1865(12):130016.
BACKGROUND: Garcinia brasiliensis is a species native to the Amazon forest. The white mucilaginous pulp is used in folk medicine as a wound healing agent and for peptic ulcer, urinary, and tumor disease treatments. The activity of the proprotein convertases (PCs) Subtilisin/Kex is associated with the development of viral, bacterial and fungal infections, osteoporosis, hyperglycemia, atherosclerosis, cardiovascular, neurodegenerative and neoplastic diseases. METHODS: Morelloflavone (BF1) and semisynthetic biflavonoid (BF2, 3 and 4) from Garcinia brasiliensis were tested as inhibitor of PCs Kex2, PC1/3 and Furin, and determined IC50, Ki, human proinflammatory cytokines secretion in Caco-2 cells, mechanism of inhibition, and performed molecular docking studies. RESULTS: Biflavonoids were more effective in the inhibition of neuroendocrine PC1/3 than mammalian Furin and fungal Kex2. BF1 presented a mixed inhibition mechanism for Kex2 and PC1, and competitive inhibition for Furin. BF4 has no good interaction with Kex2 and Furin since carboxypropyl groups results in steric hindrance to ligand-protein interactions. Carboxypropyl groups of BF4 promote steric hindrance with Kex2 and Furin, but effective in the affinity of PC1/3. BF4 was more efficient at inhibiting PCl/3 (IC50 = 1.13 muM and Ki = 0,59 muM, simple linear competitive mechanism of inhibition) than Kex2, Furin. Also, our results strongly suggested that BF4 also inhibits the endogenous cellular PC1/3 activity in Caco-2 cells, since PC1/3 inhibition by BF4 causes a large increase in IL-8 and IL-1beta secretion in Caco-2 cells. CONCLUSIONS: BF4 is a potent and selective inhibitor of PC1/3. GENERAL SIGNIFICANCE: BF4 is the best candidate for further clinical studies on inhibition of PC1/3.
Bioactivity of natural biflavonoids in metabolism-related disease and cancer therapies.[Pubmed:33667686]
Pharmacol Res. 2021 May;167:105525.
Natural biflavonoids, such as amentoflavone, bilobetin, ginkgetin, isoginkgetin, taiwaniaflavone, Morelloflavone, delicaflavone, hinokiflavone, and other derivatives (~ 40 biflavonoids), are isolated from Selaginella sp., Ginkgo biloba, Garcinia sp., and several other species of plants. They are able to exert therapeutic benefits by regulating several proteins/enzymes (PPAR-gamma, CCAAT/enhancer-binding protein alpha [C/EBPalpha], STAT5, pancreatic lipase, PTP1B, fatty acid synthase, alpha-glucosidase [AG]) and insulin signaling pathways (via PI3K-AKT), which are linked to metabolism, cell growth, and cell survival mechanisms. Deregulated insulin signaling can cause complications of obesity and diabetes, which can lead to cognitive disorders such as Alzheimer's, Parkinson's, and dementia; therefore, the therapeutic benefits of these biflavones in these areas are highlighted. Since biflavonoids have shown potential to regulate metabolism, growth- and survival-related protein/enzymes, their relation to tumor growth and metastasis of cancer associated with angiogenesis are highlighted. The translational role of biflavones in cancer with respect to the inhibition of metabolism-related processes/pathways, enzymes, or proteins, such as STAT3/SHP-1/PTEN, kinesins, tissue kallikreins, aromatase, estrogen, protein modifiers, antioxidant, autophagy, and apoptosis induction mechanisms, are discussed. Finally, considering their observed bioactivity potential, oral bioavailability studies of biflavones and related clinical trials are outlined.
Natural biflavonoids as potential therapeutic agents against microbial diseases.[Pubmed:33493916]
Sci Total Environ. 2021 May 15;769:145168.
Microbes broadly constitute several organisms like viruses, protozoa, bacteria, and fungi present in our biosphere. Fast-paced environmental changes have influenced contact of human populations with newly identified microbes resulting in diseases that can spread quickly. These microbes can cause infections like HIV, SARS-CoV2, malaria, nosocomial Escherichia coli, methicillin-resistant Staphylococcus aureus (MRSA), or Candida infection for which there are no available vaccines/drugs or are less efficient to prevent or treat these infections. In the pursuit to find potential safe agents for therapy of microbial infections, natural biflavonoids like amentoflavone, tetrahydroamentoflavone, ginkgetin, bilobetin, Morelloflavone, agathisflavone, hinokiflavone, Garcinia biflavones 1 (GB1), Garcinia biflavones 2 (GB2), robustaflavone, strychnobiflavone, ochnaflavone, dulcisbiflavonoid C, tetramethoxy-6,6''-bigenkwanin and other derivatives isolated from several species of plants can provide effective starting points and become a source of future drugs. These biflavonoids show activity against influenza, severe acute respiratory syndrome (SARS), dengue, HIV-AIDS, coxsackieviral, hepatitis, HSV, Epstein-Barr virus (EBV), protozoal (Leishmaniasis, Malaria) infections, bacterial and fungal infections. Some of the biflavonoids can provide antiviral and protozoal activity by inhibition of neuraminidase, chymotrypsin-like protease, DV-NS5 RNA dependant RNA polymerase, reverse transcriptase (RT), fatty acid synthase, DNA polymerase, UL54 gene expression, Epstein-Barr virus early antigen activation, recombinant cysteine protease type 2.8 (r-CPB2.8), Plasmodium falciparum enoyl-acyl carrier protein (ACP) reductase or cause depolarization of parasitic mitochondrial membranes. They may also provide anti-inflammatory therapeutic activity against the infection-induced cytokine storm. Considering the varied bioactivity of these biflavonoids against these organisms, their structure-activity relationships are derived and wherever possible compared with monoflavones. Overall, this review aims to highlight these natural biflavonoids and briefly discuss their sources, reported mechanism of action, pharmacological uses, and comment on resistance mechanism, flavopiridol repurposing and the bioavailability aspects to provide a starting point for anti-microbial research in this area.
Modulation of the Drug Resistance by Platonia insignis Mart. Extract, Ethyl Acetate Fraction and Morelloflavone/Volkensiflavone (Biflavonoids) in Staphylococcus aureus Strains Overexpressing Efflux Pump Genes.[Pubmed:32445452]
Curr Drug Metab. 2021;22(2):114-122.
BACKGROUND: Microbial resistance to antibiotics is a global public health problem, which requires urgent attention. Platonia insignis is a native species from the eastern Brazilian Amazon, used in the treatment of burns and wounds. OBJECTIVES: To evaluate the antimicrobial activity of the hydroalcoholic extract of P. insignis (PIHA), the ethyl acetate fraction (PIAE), and its subfraction containing a mixture of biflavonoids (BF). Moreover, the effect of these natural products on the antibiotic activity against S. aureus strains overexpressing efflux pump genes was also evaluated. METHODS: Minimal inhibitory concentrations were determined against different species of microorganisms. To evaluate the modulatory effect on the Norfloxacin-resistance, the MIC of this antibiotic was determined in the absence and presence of the natural products at subinhibitory concentrations. Inhibition of the EtBr efflux assays were conducted in the absence or presence of natural products. RESULTS: PIHA showed a microbicidal effect against S. aureus and C. albicans, while PIAE was bacteriostatic for S. aureus. PIAE and BF at subinhibitory concentrations were able to reduce the MIC of Norfloxacin acting as modulating agents. BF was able to inhibit the efflux of EtBr efflux in S. aureus strains overexpressing specific efflux pump genes. CONCLUSION: P. inignisis, a source of efflux pump inhibitors, including volkensiflavone and Morelloflavone, which were able to potentiate the Norfloxacin activity by NorA inhibition, being also able to inhibit QacA/B, TetK and MsrA. Volkensiflavone and Morelloflavone could be used as an adjuvant in the antibiotic therapy of multidrug resistant S. aureus strains overexpressing efflux pumps.
Physiological and Metabolic Effects of Yellow Mangosteen (Garcinia dulcis) Rind in Rats with Diet-Induced Metabolic Syndrome.[Pubmed:31906096]
Int J Mol Sci. 2019 Dec 31;21(1). pii: ijms21010272.
Metabolic syndrome is a cluster of disorders that increase the risk of cardiovascular disease and diabetes. This study has investigated the responses to rind of yellow mangosteen (Garcinia dulcis), usually discarded as waste, in a rat model of human metabolic syndrome. The rind contains higher concentrations of phytochemicals (such as garcinol, Morelloflavone and citric acid) than the pulp. Male Wistar rats aged 8-9 weeks were fed either corn starch diet or high-carbohydrate, high-fat diet for 16 weeks, which were supplemented with 5% freeze-dried G. dulcis fruit rind powder during the last 8 weeks. We characterised metabolic, cardiovascular, liver and gut microbiota parameters. High-carbohydrate, high-fat diet-fed rats developed abdominal obesity, hypertension, increased left ventricular diastolic stiffness, decreased glucose tolerance, fatty liver and reduced Bacteroidia with increased Clostridia in the colonic microbiota. G. dulcis fruit rind powder attenuated these changes, improved cardiovascular and liver structure and function, and attenuated changes in colonic microbiota. G. dulcis fruit rind powder may be effective in metabolic syndrome by appetite suppression, inhibition of inflammatory processes and increased fat metabolism, possibly related to changes in the colonic microbiota. Hence, we propose the use of G. dulcis fruit rind as a functional food to ameliorate symptoms of metabolic syndrome.
Aromatase (CYP19) inhibition by biflavonoids obtained from the branches of Garcinia gardneriana (Clusiaceae).[Pubmed:31393836]
Z Naturforsch C J Biosci. 2019 Sep 25;74(9-10):279-282.
Overexpression of aromatase in breast cancer cells may substantially influence its progression and maintenance. In this sense, the inhibition of aromatase is a key target for the treatment of breast cancer in postmenopausal women. Although several flavonoids had already demonstrated the capacity of inhibiting aromatase activity, the role of biflavonoids as aromatase inhibitors is poorly studied. In this work, the biflavonoids isolated from Garcinia gardneriana, Morelloflavone (1), Gb-2a (2) and Gb-2a-7-O-glucose (3) were submitted to in vitro assay to evaluate the aromatase modulatory effect. As results, it was demonstrated that all biflavonoids were able to inhibit the enzyme, with IC50 values ranging from 1.35 to 7.67 muM. This demonstrates that biflavonoids are an important source of scaffolds for the development of new aromatase inhibitors, focusing on the development of new anticancer agents.
Bacupari (Garcinia brasiliensis) extract modulates intestinal microbiota and reduces oxidative stress and inflammation in obese rats.[Pubmed:31229073]
Food Res Int. 2019 Aug;122:199-208.
The objective of this study was to evaluate the effects of an ethanolic extract of the bark of bacupari (Garcinia brasiliensis - EEB) on the abundance of intestinal microbiota, concentration of short-chain fatty acids (SCFAs), oxidative stress, and inflammation in obese rats fed a high-fat diet (HFD). Male Wistar rats were divided into three groups: an HFD-fed obese control group, a group fed HFD plus EEB (BHFD) at a dose of 300mg per animal per day (42mg 7-epiclusianone and 10.76mg Morelloflavone), and a lean control group fed an AIN-93M diet for 8weeks. EEB decreased (p<0.05) the abundance of organisms belonging to the phyla Firmicutes and Proteobacteria, and increased (p<0.05) the concentration of propionic acid. Liver concentrations of malondialdehyde, nitric oxide, resistin, and p65 nuclear factor-kappa B p65(NF-kappaB) decreased (p<0.05), while the expression of heat shock protein (HSP)72 and catalase increased (p<0.05) with the consumption of EEB. Moreover, computational molecular modeling studies involving molecular docking between the main constituents of EEB, 7-epiclusianone and Morelloflavone, and NF-kappaB suggested its inhibitory activity, thus corroborating the experimental results. The consumption of EEB may therefore be a promising strategy for the beneficial dietary modulation of the intestinal ecosystem, thereby countering oxidative stress and inflammation in obese rats. This activity is attributable to the presence of bioactive compounds that act individually or synergistically in the scavenging of free radicals or in the inflammatory process.
Chemometric classification of Garcinia madruno raw material: Impact of the regional origin and ripeness stage of a neotropical exotic species.[Pubmed:31151614]
Food Chem. 2019 Sep 30;293:291-298.
Garcinia madruno is a neotropical tree characterized by its exotic fruit and its functional compounds. The aim of this study was to evaluate the expression and variability of the chemical markers of G. madruno according to the part of the plant used, the origin and the ripeness stage by applying chemometric tools. A total of 167 samples were evaluated, and 27 compounds were quantified per sample. The expression of amentoflavone, Morelloflavone-type biflavonoids and polyisoprenylated benzophenones (PIBs) promoted intergroup differentiation, whereas the expression of GB-2a-type biflavonoids promoted intragroup cluster generation. Epicarp was the main source of biflavonoids and the secondary source of PIBs, with values up to 25% in some individuals. The origin of the fruit significantly impacted the expression of metabolites, whereas the ripeness stage did not. The results indicate that epicarp is a good source of functional compounds and, with appropriately agronomic development, could be improved even more.
Insights into the Molecular Mechanisms of Eg5 Inhibition by (+)-Morelloflavone.[Pubmed:30995725]
Pharmaceuticals (Basel). 2019 Apr 16;12(2). pii: ph12020058.
(+)-Morelloflavone (MF) is an antitumor biflavonoid that is found in the Garcinia species. Recently, we reported MF as a novel inhibitor of ATPase and microtubules-gliding activities of the kinesin spindle protein (Eg5) in vitro. Herein, we provide dynamical insights into the inhibitory mechanisms of MF against Eg5, which involves binding of the inhibitor to the loop5/alpha2/alpha3 allosteric pocket. Molecular dynamics simulations were carried out for 100 ns on eight complexes: Eg5-Adenosine diphosphate (Eg5-ADP), Eg5-ADP-S-trityl-l-cysteine (Eg5-ADP-STLC), Eg5-ADP-ispinesib, Eg5-ADP-MF, Eg5-Adenosine triphosphate (Eg5-ATP), Eg5-ATP-STLC, Eg5-ATP-ispinesib, and Eg5-ATP-MF complexes. Structural and energetic analyses were done using Umbrella sampling, Molecular Mechanics Poisson-Boltzmann Surface Area (MM/PBSA) method, GROMACS analysis toolkit, and virtual molecular dynamics (VMD) utilities. The results were compared with those of the known Eg5 inhibitors; ispinesib, and STLC. Our data strongly support a stable Eg5-MF complex, with significantly low binding energy and reduced flexibility of Eg5 in some regions, including loop5 and switch I. Furthermore, the loop5 Trp127 was trapped in a downward position to keep the allosteric pocket of Eg5 in the so-called "closed conformation", comparable to observations for STLC. Altered structural conformations were also visible within various regions of Eg5, including switch I, switch II, alpha2/alpha3 helices, and the tubulin-binding region, indicating that MF might induce modifications in the Eg5 structure to compromise its ATP/ADP binding and conversion process as well as its interaction with microtubules. The described mechanisms are crucial for understanding Eg5 inhibition by MF.
Morelloflavone as a novel inhibitor of mitotic kinesin Eg5.[Pubmed:30785183]
J Biochem. 2019 Aug 1;166(2):129-137.
Among 40 plant-derived biflavonoids with inhibitory potential against Eg5, Morelloflavone from Garcinia dulcis leaves was selected for further testing based on in silico analysis of binding modes, molecular interactions, binding energies and functional groups that interact with Eg5. Computational models predicted that Morelloflavone binds the putative allosteric pocket of Eg5, within the cavity surrounded by amino acid residues of Ile-136, Glu-116, Glu-118, Trp-127, Gly-117, Ala-133, Glu-215, Leu-214 and Tyr-211. Binding energy was -8.4 kcal/mol, with a single hydrogen bond formed between Morelloflavone and Tyr-211. The binding configuration was comparable to that of a reference inhibitor, S-trityl-L-cysteine. Subsequent biochemical analysis in vitro confirmed that Morelloflavone inhibited both the basal and microtubule-activated ATPase activity of Eg5 in a manner that does not compete with ATP binding. Morelloflavone also suppressed Eg5 gliding along microtubules. These results suggest that Morelloflavone binds the allosteric binding site in Eg5 and thereby inhibits ATPase activity and motor function of Eg5.
Bacupari peel extracts (Garcinia brasiliensis) reduces the biometry, lipogenesis and hepatic steatosis in obese rats.[Pubmed:30361013]
Food Res Int. 2018 Dec;114:169-177.
The aim was to evaluate the effect of the ethanol extract of bacupari peel (EEB) on biometric measurements, hepatic lipogenesis and progression of non-alcoholic fatty liver disease (NAFLD) in obese Wistar rats. Chemical analysis of the bacupari peel extract identified 7-epiclusianone as the major constituent (140.02mg/g) followed by Morelloflavone (35.86mg/g). Animals treated with high fat diet plus EEB (BHFD) reduced body mass index (BMI), liver weight and hepatosomatic index in relation to the obese control. The food intake was similar between hyperlipid group (HFD) groups with or without EEB. However, the normal control group (AIN-93M) presented higher food intake and lower final weight compared to the obese control (HFD). The PPAR-alpha, CPT-1a and the ADIPOR2 genes expressions, and the concentration of the PPAR-alpha and the adiponectin protein level increased in the BHFD group in relation to the obese control. The EEB promoted reduction of the SREBP-1c gene expression and the percentage of hepatic fat and the degree of steatosis in relation to HFD. It was concluded that EEB showed a protective effect on NAFLD, as it promoted a reduction in BMI, induced lipid oxidation, reduced lipogenesis and hepatic steatosis. Moreover, our results suggest an interaction that can lead to an agonist activity of the EEB to the PPAR-alpha receptor.
Bioactive properties and phytochemical assessment of Bacupari-anao (Garcinia brasiliensis Mart.) leaves native to Rondonia, Brazil.[Pubmed:30302477]
Food Funct. 2018 Nov 14;9(11):5621-5628.
Leaf fractions of Garcinia brasiliensis were evaluated concerning their antioxidant, antimicrobial, anti-inflammatory and cytotoxic properties, and the most active fraction was then fully characterized regarding its phenolic composition using HPLC-DAD-ESI/MSn. The ethyl acetate fraction from partitioning of the methanolic leaf extract revealed a strong antioxidant activity that was comparable to Trolox, the positive control. This fraction was also able to show a significant antimicrobial activity against Gram-positive and Gram-negative bacteria and the fungus Candida albicans. However, the dichloromethane fraction was found to present the highest anti-inflammatory (83 +/- 9 mug mL-1) and cytotoxic activities, thus presenting slight toxicity using a non-tumor cell line. Regarding the phenolic profile, the ethyl acetate fraction presented twelve flavonoids, with Morelloflavone-7''-O-glucoside (52.1 +/- 0.4 mg g-1) and gardinia biflavonoid 2a glucoside (27.5 +/- 0.2 mg g-1) being the major compounds identified. These results indicate that leaves of G. brasiliensis might be a potential source of natural biomolecules for pharmaceutical and medicinal applications.
Guttiferone BL with antibacterial activity from the fruits of Allanblackia gabonensis.[Pubmed:29683342]
Nat Prod Res. 2019 Sep;33(18):2638-2646.
Allanblackia genus, an endless source of bioactive compounds, was investigated for its antibacterial properties. The chemical study of the methanol extract from the fruits of Allanblackia gabonensis resulted in the isolation of the undescribed guttiferone BL (1) along with the known kaempferol (2), Morelloflavone (3), Morelloflavone 7''-O-beta-D-glucopyranoside (4), beta-sitosterol 3-O-beta-D-glucopyranoside and beta-sitosterol. Their structures were determined using spectrometry and spectroscopic techniques. The antibacterial activity was evaluated against five Gram-negative and two Gram-positive strains using a broth micro-dilution method. Compounds displayed low to significant activity against the tested bacterial strains with MICs ranging from 8 to 512 mug/mL. Morelloflavone (3) presented significant activity against E. coli ATCC8739 (MIC = 8 mug/mL) while guttiferone BL (1) exhibited low activity (MICs = 256-512 mug/mL) against all the tested strains. The crude extract also had moderate to significant activity against the tested bacterial strains.
Effects of biflavonoids from Garcinia madruno on a triple transgenic mouse model of Alzheimer's disease.[Pubmed:29229355]
Pharmacol Res. 2018 Mar;129:128-138.
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that is pathologically characterized by the deposition of beta-amyloid (betaA) peptides in senile plaques and neurofibrillary tangles in the brain. Flavonoids have recently been used to prevent and treat a variety of neurodegenerative diseases, but little is known about bioflavonoids. In this study, we evaluate whether a biflavonoid fraction (BF) exerts neuroprotective effects on an aged triple transgenic mouse mode of AD (3xTg-AD). Then, 21-24-month-old 3xTg AD mice were i.p. injected with 25mg/kg of a BF from Garcinia madruno composed of Morelloflavone (65%), volkensiflavone (12%), GB 2a (11%), fukugiside (6%) and amentoflavone (0.4%) every 48h for 3 months. The BF treatment reduced betaA deposition in different regions of the brain (the hippocampus, entorhinal cortex and amygdala), reduced betaA1-40 and betaA1-42 levels, BACE1-mediated cleavage of APP (CTFbeta), tau pathology, astrogliosis and microgliosis in the brains of aged 3xTg-AD mice. Although the BF treatment weakly improved learning, animals treated with BF spent more time in the open arms of the elevated plus maze test and displayed greater risk assessment behavior than the control groups. In summary, the BF reverses histopathological hallmarks and reduces emotional disorders in the 3xTg-AD mouse model, suggesting that the biflavonoids from G. madruno represent a potential natural therapeutic option for AD if its bioavailability is improved.


