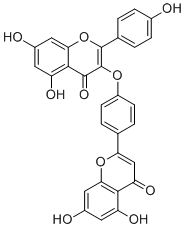
DelicaflavoneCAS# 343569-15-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 343569-15-3 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Yellow powder |
| Formula | C30H18O10 | M.Wt | 538.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Delicaflavone Dilution Calculator

Delicaflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.857 mL | 9.2851 mL | 18.5701 mL | 37.1402 mL | 46.4253 mL |
| 5 mM | 0.3714 mL | 1.857 mL | 3.714 mL | 7.428 mL | 9.2851 mL |
| 10 mM | 0.1857 mL | 0.9285 mL | 1.857 mL | 3.714 mL | 4.6425 mL |
| 50 mM | 0.0371 mL | 0.1857 mL | 0.3714 mL | 0.7428 mL | 0.9285 mL |
| 100 mM | 0.0186 mL | 0.0929 mL | 0.1857 mL | 0.3714 mL | 0.4643 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Luteolin 5-methyl ether
Catalog No.:BCN0699
CAS No.:58115-29-0
- Violanone
Catalog No.:BCN0698
CAS No.:52250-38-1
- Luteolin 5,3'-dimethyl ether
Catalog No.:BCN0697
CAS No.:62346-14-9
- (2S)-Pinocembrin 7-O-[2''-O-(5'''-O-trans-cinnamoyl)-β-D-apiofuranosyl]-β-D-glucoside
Catalog No.:BCN0696
CAS No.:773899-29-9
- Kayaflavone
Catalog No.:BCN0695
CAS No.:481-45-8
- E,Z-Platanoside
Catalog No.:BCN0694
CAS No.:1197343-17-1
- Quercetin 4'-O-galactoside
Catalog No.:BCN0693
CAS No.:381728-34-3
- Tamarixin
Catalog No.:BCN0692
CAS No.:27542-39-8
- Morelloflavone
Catalog No.:BCN0691
CAS No.:16851-21-1
- Platanoside
Catalog No.:BCN0690
CAS No.:133740-25-7
- 2''-O-E-p-Coumaroylafzelin
Catalog No.:BCN0689
CAS No.:151455-10-6
- 3,5,7-Trihydroxy-8-methoxyflavone
Catalog No.:BCN0688
CAS No.:5928-42-7
- 5,6-Dihydroxy-7,8-dimethoxyflavone
Catalog No.:BCN0701
CAS No.:76844-65-0
- Shanciol B
Catalog No.:BCN0702
CAS No.:208106-53-0
- Ochnaflavone
Catalog No.:BCN0703
CAS No.:50276-96-5
- 3',3'''-Biapigenin
Catalog No.:BCN0704
CAS No.:151455-26-4
- Chrysocauloflavone I
Catalog No.:BCN0705
CAS No.:899789-51-6
- 6,7,4'-Trihydroxyflavanone
Catalog No.:BCN0706
CAS No.:189689-31-4
- Procyanidin B2 3,3'-di-O-gallate
Catalog No.:BCN0707
CAS No.:79907-44-1
- 2'',3''-Dihydro-3',3'''-biapigenin
Catalog No.:BCN0708
CAS No.:151455-25-3
- 3',3'''-Binaringenin
Catalog No.:BCN0709
CAS No.:145399-99-1
- 2'',3''-Dihydroochnaflavone
Catalog No.:BCN0710
CAS No.:340997-02-6
- Procyanidin B5
Catalog No.:BCN0711
CAS No.:12798-57-1
- Procyanidin B2 3''-O-gallate
Catalog No.:BCN0712
CAS No.:73086-04-1
In vitro antibacterial activity of Loxostylis alata extracts and isolated compounds against Salmonella species.[Pubmed:33849505]
BMC Complement Med Ther. 2021 Apr 13;21(1):121.
BACKGROUND: Owing to antibiotic resistance, alternative antimicrobials from medicinal plants are receiving attention as leads for anti-infective agents. This study aimed to investigate selected tree species and their constituents for activity against bacterial foodborne pathogens, particularly Salmonella serovars. METHODS: Antibacterial activity of ten plant species was determined by serial microdilution against bacteria implicated in causing gastrointestinal ailments. Active compounds were isolated from Loxostylis alata using bioassay-guided fractionation. Antioxidant activity was determined using free-radical scavenging assays. Cytotoxicity and genotoxicity of the extracts was ascertained on Vero cells, and using the Ames assay respectively. RESULTS: Extracts had low to moderate MIC values from 0.04 to 2.5 mg/mL. Protorhus longifolia and Loxostylis alata were most active and L. alata had the highest selectivity index value (2.51) against Salmonella Typhimurium, as well as high antioxidant activity. Cytotoxicity values ranged from 0.02 to 0.47 mg/mL, while tested extracts were not genotoxic. Bioactive compounds isolated from L. alata included Delicaflavone and a polymethoxyflavone. CONCLUSIONS: The Loxostylis alata leaf extract had strong activity against Salmonella serovars but isolated compounds were less active, indicating likely synergistic effects. Extracts of L. alata are promising candidates for development of antimicrobial preparations or food additives against microbial contamination.
Bioactivity of natural biflavonoids in metabolism-related disease and cancer therapies.[Pubmed:33667686]
Pharmacol Res. 2021 May;167:105525.
Natural biflavonoids, such as amentoflavone, bilobetin, ginkgetin, isoginkgetin, taiwaniaflavone, morelloflavone, Delicaflavone, hinokiflavone, and other derivatives (~ 40 biflavonoids), are isolated from Selaginella sp., Ginkgo biloba, Garcinia sp., and several other species of plants. They are able to exert therapeutic benefits by regulating several proteins/enzymes (PPAR-gamma, CCAAT/enhancer-binding protein alpha [C/EBPalpha], STAT5, pancreatic lipase, PTP1B, fatty acid synthase, alpha-glucosidase [AG]) and insulin signaling pathways (via PI3K-AKT), which are linked to metabolism, cell growth, and cell survival mechanisms. Deregulated insulin signaling can cause complications of obesity and diabetes, which can lead to cognitive disorders such as Alzheimer's, Parkinson's, and dementia; therefore, the therapeutic benefits of these biflavones in these areas are highlighted. Since biflavonoids have shown potential to regulate metabolism, growth- and survival-related protein/enzymes, their relation to tumor growth and metastasis of cancer associated with angiogenesis are highlighted. The translational role of biflavones in cancer with respect to the inhibition of metabolism-related processes/pathways, enzymes, or proteins, such as STAT3/SHP-1/PTEN, kinesins, tissue kallikreins, aromatase, estrogen, protein modifiers, antioxidant, autophagy, and apoptosis induction mechanisms, are discussed. Finally, considering their observed bioactivity potential, oral bioavailability studies of biflavones and related clinical trials are outlined.
Hinokiflavone and Related C-O-C-Type Biflavonoids as Anti-cancer Compounds: Properties and Mechanism of Action.[Pubmed:33534099]
Nat Prod Bioprospect. 2021 Aug;11(4):365-377.
Biflavonoids are divided in two classes: C-C type compounds represented by the dimeric compound amentoflavone and C-O-C-type compounds typified by hinokiflavone (HNK) with an ether linkage between the two connected apigenin units. This later sub-group of bisflavonyl ethers includes HNK, ochnaflavone, Delicaflavone and a few other dimeric compounds, found in a variety of plants, notably Selaginella species. A comprehensive review of the anticancer properties and mechanism of action of HNK is provided, to highlight the anti-proliferative and anti-metastatic activities of HNK and derivatives, and HNK-containing plant extracts. The anticancer effects rely on the capacity of HNK to interfere with the ERK1-2/p38/NFkappaB signaling pathway and the regulation of the expression of the matrix metalloproteinases MMP-2 and MMP-9 (with a potential direct binding to MMP-9). In addition, HNK was found to function as a potent modulator of pre-mRNA splicing, inhibiting the SUMO-specific protease SENP1. As such, HNK represents a rare SENP1 inhibitor of natural origin and a scaffold to design synthetic compounds. Oral formulations of HNK have been elaborated to enhance its solubility, to facilitate the compound delivery and to enhance its anticancer efficacy. The review shed light on the anticancer potential of C-O-C-type biflavonoids and specifically on the pharmacological profile of HNK. This compound deserves further attention as a regulator of pre-mRNA splicing, useful to treat cancers (in particular hepatocellular carcinoma) and other human pathologies.
Molecular mechanism and pharmacokinetics of flavonoids in the treatment of resistant EGF receptor-mutated non-small-cell lung cancer: A narrative review.[Pubmed:33450055]
Br J Pharmacol. 2021 Mar;178(6):1388-1406.
Here, we review the molecular mechanism and pharmacokinetics of flavonoids in the treatment of resistant EGF receptor (EGFR)-mutated non-small-cell lung cancer (NSCLC) and particularly the possible mechanism(s) of Delicaflavone, a biflavonoid extracted from Selaginella doederleinii Hieron. EGFR TK inhibitors (EGFR-TKI) are ubiquitously used in the treatment of NSCLC bearing EGFR mutations. However, patients treated with EGFR-TKI inevitably and continuously develop resistance. In laboratory studies, flavonoids, as potential adjuvants for cancer chemotherapy, exhibited anti-cancer properties such as inhibition of chemoresistance by interference with ABC transporters-induced drug efflux, curbing of c-MET amplification, or reversal of T790M mutation-mediated resistance. The current review aims at summarizing the association between the anti-cancer potentials of flavonoids and their possible regulatory roles in certain types of mutation that could trigger EGFR-TKI resistance in NSCLC. Potential practical applications of these phytochemicals, as well as the relevant pharmacokinetics, are also discussed.
Delicaflavone Reverses Cisplatin Resistance via Endoplasmic Reticulum Stress Signaling Pathway in Non-Small Cell Lung Cancer Cells.[Pubmed:33116611]
Onco Targets Ther. 2020 Oct 13;13:10315-10322.
Background: The incidence and mortality of lung cancer continue to increase around the world; in 2018, new lung cancer cases accounted for 11.6% of all cancer cases, and lung cancer deaths accounted for 18.4% of cancer deaths. Cisplatin (DDP) is a first-line chemotherapy drug for lung cancer; however, DDP resistance can lead to a poor prognosis in patients with lung cancer. Therefore, reversing DDP resistance is a treatment goal. Materials and Methods: Cell counting kit-8 (CCK8) assays, wound healing analyses, Transwell assays, in vitro tumor xenografts, and flow cytometry were used to detect the proliferation, migration, invasion, and apoptosis of multidrug resistant A549/DDP and PC9/DDP cells, respectively. Western blot was performed to detect protein levels of cleaved caspase-3, CHOP, and GRP78. Results: Delicaflavone inhibited DDP resistance of lung cancer cells and decreased proliferation in a dose- and time-dependent manner. It also decreased migration and invasion and enhanced apoptosis. Western blots showed that Delicaflavone overcame DDP resistance by increasing the expression of GRP78 and CHOP and the apoptosis-related protein cleaved caspase-3. Conclusion: Delicaflavone can reverse DDP resistance in A549/DDP and PC9/DDP cells by inhibiting cell proliferation and migration and enhancing apoptosis and cleaved caspase-3 levels by increasing the expression of CHOP and GRP78 protein via the endoplasmic reticular stress pathway. It could be a useful therapeutic adjunct to treat DDP-resistant lung cancer.
Improved solubility, dissolution rate, and oral bioavailability of main biflavonoids from Selaginella doederleinii extract by amorphous solid dispersion.[Pubmed:32037895]
Drug Deliv. 2020 Dec;27(1):309-322.
Amentoflavone, robustaflavone, 2'',3''-dihydro-3',3'''-biapigenin, 3',3'''-binaringenin, and Delicaflavone are five major hydrophobic components in the total biflavonoids extract from Selaginella doederleinii (TBESD) that display favorable anticancer properties. The purpose of this study was to develop a new oral delivery formulation to improve the solubilities, dissolution rates, and oral bioavailabilities of the main ingredients in TBESD by the solid dispersion technique. Solid dispersions of TBESD with various hydrophilic polymers were prepared, and different technologies were applied to select the suitable carrier and method. TBESD amorphous solid dispersion (TBESD-ASD) with polyvinylpyrrolidone K-30 was successfully prepared by the solvent evaporation method. The physicochemical properties of TBESD-ASD were investigated by scanning electron microscopy, differential scanning calorimetry, and Fourier-transform infrared spectroscopy. As a result, TBESD was found to be molecularly dispersed in the amorphous carrier. The solubilities and dissolution rates of all five ingredients in the TBESD-ASD were significantly increased (nearly 100% release), compared with raw TBESD. Meanwhile, TBESD-ASD showed good preservation stability for 3 months under accelerated conditions of 40 degrees C and 75% relative humidity. A subsequent pharmacokinetic study in rats revealed that Cmax and AUC0-t of all five components were significantly increased by the solid dispersion preparation. An in vivo study clearly revealed that compared to raw TBESD, a significant reduction in tumor size and microvascular density occurred after oral administration of TBESD-ASD to xenograft-bearing tumor mice. Collectively, the developed TBESD-ASD with the improved solubility, dissolution rates and oral bio-availabilities of the main ingredients could be a promising chemotherapeutic agent for cancer treatment.
Proliposomes for oral delivery of total biflavonoids extract from Selaginella doederleinii: formulation development, optimization, and in vitro-in vivo characterization.[Pubmed:31692515]
Int J Nanomedicine. 2019 Aug 20;14:6691-6706.
Purpose: Amentoflavone, robustaflavone, 2'',3''-dihydro-3',3'''-biapigenin, 3',3'''-binaringenin and Delicaflavone are five major active ingredients in the total biflavonoids extract from Selaginella doederleinii (TBESD) with favorable anticancer properties. However, the natural-derived potent antitumor agent of TBESD is undesirable due to its poor solubility. The present study was to develop and optimize a proliposomal formulation of TBESD (P-TBESD) to improve its solubility, oral bioavailability and efficacy. Materials and methods: P-TBESD containing a bile salt, a protective hydrophilic isomalto-oligosaccharides (IMOs) coating, were successfully prepared by thin film dispersion-sonication method. The physicochemical and pharmacokinetic properties of P-TBESD were characterized, and the antitumor effect was evaluated using the HT-29 xenograft-bearing mice models in rats. Results: Compared with TBESD, the relative bioavailability of amentoflavone, robustaflavone, 2'',3''-dihydro-3',3'''-biapigenin, 3',3'''-binaringenin and Delicaflavone from P-TBESD were 669%, 523%, 761%, 955% and 191%, respectively. The results of pharmacodynamics demonstrated that both TBESD and P-TBESD groups afforded antitumor effect without systemic toxicity, and the antitumor effect of P-TBESD was significantly superior to that of raw TBESD, based on the tumor growth inhibition and histopathological examination. Conclusion: Hence, IMOs-modified proliposomes have promising potential for TBESD solving the problem of its poor solubility and oral bioavailability, which can serve as a practical oral preparation for TBESD in the future cancer therapy.
Delicaflavone induces ROS-mediated apoptosis and inhibits PI3K/AKT/mTOR and Ras/MEK/Erk signaling pathways in colorectal cancer cells.[Pubmed:31669234]
Biochem Pharmacol. 2020 Jan;171:113680.
Colorectal cancer (CRC) is one of the most common malignant tumors worldwide and tends to have drug resistance. Delicaflavone (DLF), a novel anticancer agent of biflavonoid from Selaginella doederleinii Hieron, showed strong anti-CRC activities, which has not yet been reported. In this study, we investigated the effects and possible anti-CRC mechanism of DLF in vitro and in vivo. It was shown that DLF significantly inhibited the cells viability and induced G2/M phase arrest, apoptosis, the loss of mitochondrial membrane potential (Deltapsim), generation of ROS and increase of intracellular Ca(2+) in HT29 and HCT116 cells by MTT assay, TEM, flow cytometry and inverted fluorescence microscope. Western blot and qPCR assays results further confirmed DLF induced caspase-dependent apoptosis and inhibited PI3K/AKT/mTOR and Ras/MEK/Erk signaling pathways in CRC cells. Meanwhile, DLF significantly suppressed the tumor growth via activation of Caspase-9 and Caspase-3 protein and decrease of ki67 and CD34 protein without apparent side effects in vivo. In summary, these results indicated DLF induced ROS-mediated cell cycle arrest and apoptosis through ER stress and mitochondrial pathway accompanying with the inhibition of PI3K/AKT/mTOR and Ras/MEK/Erk signaling cascade. Thus DLF could be a potential therapeutic agent for CRC.
Delicaflavone induces apoptosis via mitochondrial pathway accompanying G2/M cycle arrest and inhibition of MAPK signaling cascades in cervical cancer HeLa cells.[Pubmed:31177019]
Phytomedicine. 2019 Sep;62:152973.
BACKGROUND: Cervical cancer (CCa) represents the fourth most common cause of cancer-related death in women worldwide. CCa therapy is still a major clinical challenge worldwide. Finding and developing new anti-CCa chemotherapeutic drugs is a very significant issue. Delicaflavone is a rare biflavonoid from Selaginella doederleinii Hieron, which has shown strong anti-cancer activities in our preliminary screening. PURPOSE: The present study aimed to investigate the apoptotic effect and mechanism of Delicaflavone against CCa. METHODS: In this study, the effect and potential mechanism of Delicaflavone against CCa were investigated in vitro and in vivo by MTT assay, TEM, flow cytometry, western blot assay, qPCR assay, immunofluorescence assay and the mouse xenograft tumor model. RESULTS: It was confirmed that Delicaflavone inhibited the proliferation of human CCa HeLa cells, and induced morphological changes, G2/M phase arrest and apoptosis in a dose- and time-dependent manner. HeLa cells treated with Delicaflavone showed the loss of mitochondrial membrane potential, release of Cytochrome c, activation of caspases, alteration of Bax/Bcl-2 balance, and the inhibition of MAPK signaling cascades. Furthermore, Delicaflavone significantly decreased tumor growth in a dose-dependent manner without apparent side effects in a xenograft tumor model of HeLa cells. Immunohistochemistry analysis confirmed the up-regulation of Caspase-9, Caspase-3, Bax protein and down-regulation of Bcl-2 protein in the xenografts tumors, which was consistent with the results in vitro. CONCLUSION: The results of the current study show that apoptosis is induced by the mitochondrial pathway accompanying with G2/M cycle arrest and inhibition of MAPK signaling cascades in human CCa HeLa cells, which can be used as a promising therapeutic drug for CCa.
Simultaneous quantification of five biflavonoids in rat plasma by LC-ESI-MS/MS and its application to a comparatively pharmacokinetic study of Selaginella doederleinii Hieron extract in rats.[Pubmed:29101819]
J Pharm Biomed Anal. 2018 Feb 5;149:80-88.
Selaginella doederleinii Hieron is a widely used as folk Chinese medicine for treatment of different cancers. Our previous investigations have confirmed that the total biflavonoids in ethyl acetate extract from S. doederleinii (SDEA) have favorable anticancer potentials. However, the in vivo process of its bioactive ingredients remains unknown. In this paper, a sensitive and reliable method was developed for simultaneous quantification of main five biflavonoids, including amentoflavone, robustaflavone, 2'',3''-dihydro-3',3''-biapigenin, 3',3''-binaringenin and Delicaflavone in the ethyl acetate extract of S. doederleinii (SDEA extract) in rat plasma by high-performance liquid chromatography with electrospray ionization-mass spectrometry (HPLC-ESI-MS/MS). Chromatographic separation was performed using an Ultimate((R)) XB-C18 (100x2.1mm, 3.5mum) with gradient elution of water (0.5% acetic acid) and acetonitrile at 0.2mL/min. All analytes with internal standard (chrysin) were detected using selective reaction monitoring (SRM) in negative ionization mode. The method showed a good linearity over a wide concentration range (r(2)>0.99). The limits of quantification for the biflavonoids were less than 10ng/mL. The developed method was applied to the comparatively pharmacokinetic study of the five biflavonoids after oral or intravenous administration of SDEA extract in rats. In addition, in silico assessments of permeability and solubility of these biflavonoids were also performed to understand their poor bioavailability. It is the first time to report the in vivo process profiles of the biflavonoids of SDEA extract in rats.
Delicaflavone induces autophagic cell death in lung cancer via Akt/mTOR/p70S6K signaling pathway.[Pubmed:27838742]
J Mol Med (Berl). 2017 Mar;95(3):311-322.
Searching for potential anticancer agents from natural sources is an effective strategy for developing novel chemotherapeutic agents. In this study, data supporting the in vitro and in vivo anticancer effects of Delicaflavone, a rarely occurring biflavonoid from Selaginella doederleinii, were reported. Delicaflavone exhibited favorable anticancer properties, as shown by the MTT assay and xenograft model of human non-small cell lung cancer in male BALB/c nude mice without observable adverse effect. By transmission electron microscopy with acridine orange and Cyto-ID(R)Autophagy detection dyes, Western blot analysis, and RT-PCR assay, we confirmed that Delicaflavone induces autophagic cell death by increasing the ratio of LC3-II to LC3-I, which are autophagy-related proteins, and promoting the generation of acidic vesicular organelles and autolysosomes in the cytoplasm of human lung cancer A549 and PC-9 cells in a time- and dose-dependent manner. Delicaflavone downregulated the expression of phospho-Akt, phospho-mTOR, and phospho-p70S6K in a time- and dose-dependent manner, suggesting that it induced autophagy by inhibiting the Akt/mTOR/p70S6K pathway in A549 and PC-9 cells. Delicaflavone is a potential anticancer agent that can induce autophagic cell death in human non-small cell lung cancer via the Akt/mTOR/p70S6K signaling pathway. Delicaflavone showed anti-lung cancer effects in vitro and in vivo. Delicaflavone induced autophagic cell death via Akt/mTOR/p70S6K signaling pathway. Delicaflavone did not show observable side effects in a xenograft mouse model. Delicaflavone may represent a potential therapeutic agent for lung cancer. KEY MESSAGES: Delicaflavone showed anti-lung cancer effects in vitro and in vivo. Delicaflavone induced autophagic cell death via Akt/mTOR/p70S6K signaling pathway. Delicaflavone did not show observable side effects in a xenograft mouse model. Delicaflavone may represent a potential therapeutic agent for lung cancer.


